1. Moon SJ, Kim TH, Yoon SY, Chung JH, Hwang HJ. Relationship between stage of chronic kidney disease and sarcopenia in Korean aged 40 years and older using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008-2011.
PLoS One 2015;10:e0130740.



2. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis.
Age Ageing 2019;48:16–31.



3. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment.
J Am Med Dir Assoc 2020;21:300–307.


4. Pereira RA, Cordeiro AC, Avesani CM, et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality.
Nephrol Dial Transplant 2015;30:1718–1725.


5. Kim JK, Kim SG, Oh JE, et al. Impact of sarcopenia on long-term mortality and cardiovascular events in patients undergoing hemodialysis.
Korean J Intern Med 2019;34:599–607.



6. Giglio J, Kamimura MA, Lamarca F, Rodrigues J, Santin F, Avesani CM. Association of sarcopenia with nutritional parameters, quality of life, hospitalization, and mortality rates of elderly patients on hemodialysis.
J Ren Nutr 2018;28:197–207.


7. Aly SH, Tawfik HM, Bichari WA, Alsadany MA, Banouby E, Hassan M. Sarcopenia and risk of falls in patients with chronic kidney disease, a meet of three geriatric giants.
Egypt J Geriatr Gerontol 2019;6:1–5.

8. Carrero JJ, Chmielewski M, Axelsson J, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients.
Clin Nutr 2008;27:557–564.


9. Beddhu S, Wei G, Marcus RL, Chonchol M, Greene T. Light-intensity physical activities and mortality in the United States general population and CKD subpopulation.
Clin J Am Soc Nephrol 2015;10:1145–1153.



12. Ishikawa S, Naito S, Iimori S, et al. Loop diuretics are associated with greater risk of sarcopenia in patients with non-dialysis-dependent chronic kidney disease.
PLoS One 2018;13:e0192990.



13. Zhou Y, Hellberg M, Svensson P, Höglund P, Clyne N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3-5.
Nephrol Dial Transplant 2018;33:342–348.


14. Kamijo Y, Kanda E, Ishibashi Y, Yoshida M. Sarcopenia and frailty in PD: impact on mortality, malnutrition, and inflammation.
Perit Dial Int 2018;38:447–454.



15. Abro A, Delicata LA, Vongsanim S, Davenport A. Differences in the prevalence of sarcopenia in peritoneal dialysis patients using hand grip strength and appendicular lean mass: depends upon guideline definitions.
Eur J Clin Nutr 2018;72:993–999.



16. da Silva MZ, Vogt BP, Reis NS, Caramori JC. Update of the European consensus on sarcopenia: what has changed in diagnosis and prevalence in peritoneal dialysis?
Eur J Clin Nutr 2019;73:1209–1211.



18. Fried LF, Boudreau R, Lee JS, et al. Kidney function as a predictor of loss of lean mass in older adults: health, aging and body composition study.
J Am Geriatr Soc 2007;55:1578–1584.


19. Kim H, Suzuki T, Kim M, et al. Incidence and predictors of sarcopenia onset in community-dwelling elderly Japanese women: 4-year follow-up study.
J Am Med Dir Assoc 2015;16:85.

20. Liu CK, Lyass A, Massaro JM, D’Agostino RB Sr, Fox CS, Murabito JM. Chronic kidney disease defined by cystatin C predicts mobility disability and changes in gait speed: the Framingham Offspring Study.
J Gerontol A Biol Sci Med Sci 2014;69:301–307.


21. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates.
J Gerontol A Biol Sci Med Sci 2014;69:547–558.



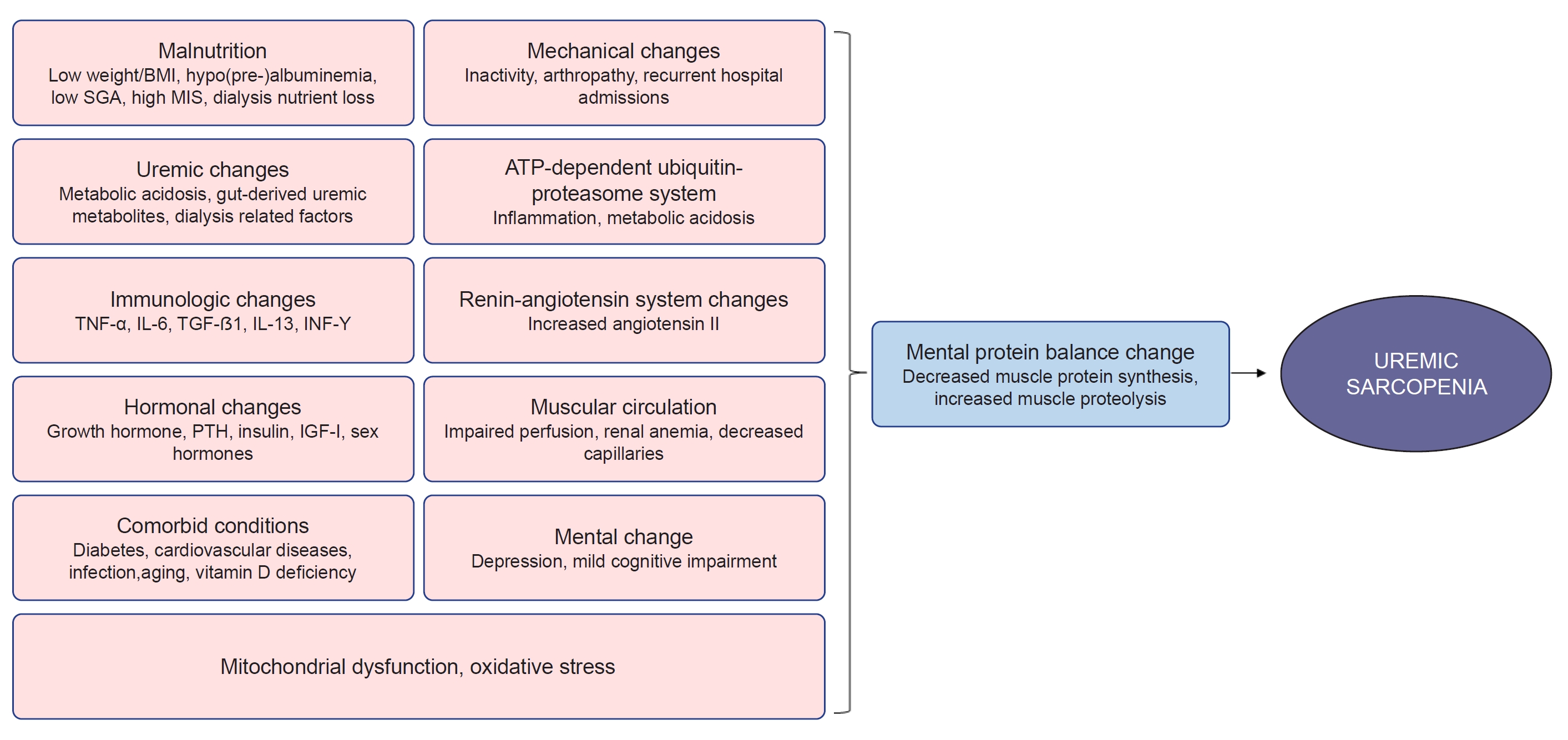
22. Fahal IH. Uraemic sarcopenia: aetiology and implications.
Nephrol Dial Transplant 2014;29:1655–1665.


23. Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training.
J Cachexia Sarcopenia Muscle 2010;1:9–21.



25. Vettoretti S, Caldiroli L, Armelloni S, Ferrari C, Cesari M, Messa P. Sarcopenia is associated with malnutrition but not with systemic inflammation in older persons with advanced CKD.
Nutrients 2019;11:1378.



26. Souza VA, Oliveira D, Barbosa SR, et al. Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors.
PLoS One 2017;12:e0176230.



27. Kim JK, Choi SR, Choi MJ, et al. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease.
Clin Nutr 2014;33:64–68.


28. Tabibi H, As’habi A, Najafi I, Hedayati M. Prevalence of dynapenic obesity and sarcopenic obesity and their associations with cardiovascular disease risk factors in peritoneal dialysis patients.
Kidney Res Clin Pract 2018;37:404–413.



29. Bataille S, Serveaux M, Carreno E, Pedinielli N, Darmon P, Robert A. The diagnosis of sarcopenia is mainly driven by muscle mass in hemodialysis patients.
Clin Nutr 2017;36:1654–1660.


30. As’habi A, Najafi I, Tabibi H, Hedayati M. Prevalence of sarcopenia and dynapenia and their determinants in iranian peritoneal dialysis patients.
Iran J Kidney Dis 2018;12:53–60.

31. Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients.
Clin J Am Soc Nephrol 2014;9:1720–1728.



32. Ren H, Gong D, Jia F, Xu B, Liu Z. Sarcopenia in patients undergoing maintenance hemodialysis: incidence rate, risk factors and its effect on survival risk.
Ren Fail 2016;38:364–371.


33. Nishikawa M, Ishimori N, Takada S, et al. AST-120 ameliorates lowered exercise capacity and mitochondrial biogenesis in the skeletal muscle from mice with chronic kidney disease via reducing oxidative stress.
Nephrol Dial Transplant 2015;30:934–942.


35. Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure.
Am J Physiol Endocrinol Metab 2000;278:E219–E225.


36. Yazdi PG, Moradi H, Yang JY, Wang PH, Vaziri ND. Skeletal muscle mitochondrial depletion and dysfunction in chronic kidney disease.
Int J Clin Exp Med 2013;6:532–539.


37. Tamaki M, Miyashita K, Wakino S, Mitsuishi M, Hayashi K, Itoh H. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase.
Kidney Int 2014;85:1330–1339.


39. Kalantar-Zadeh K, Mehrotra R, Fouque D, Kopple JD. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure.
Semin Dial 2004;17:455–465.

40. Kalantar-Zadeh K, Fouque D. Nutritional management of chronic kidney disease.
N Engl J Med 2017;377:1765–1776.


41. Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy.
J Am Soc Nephrol 2006;17:1388–1394.

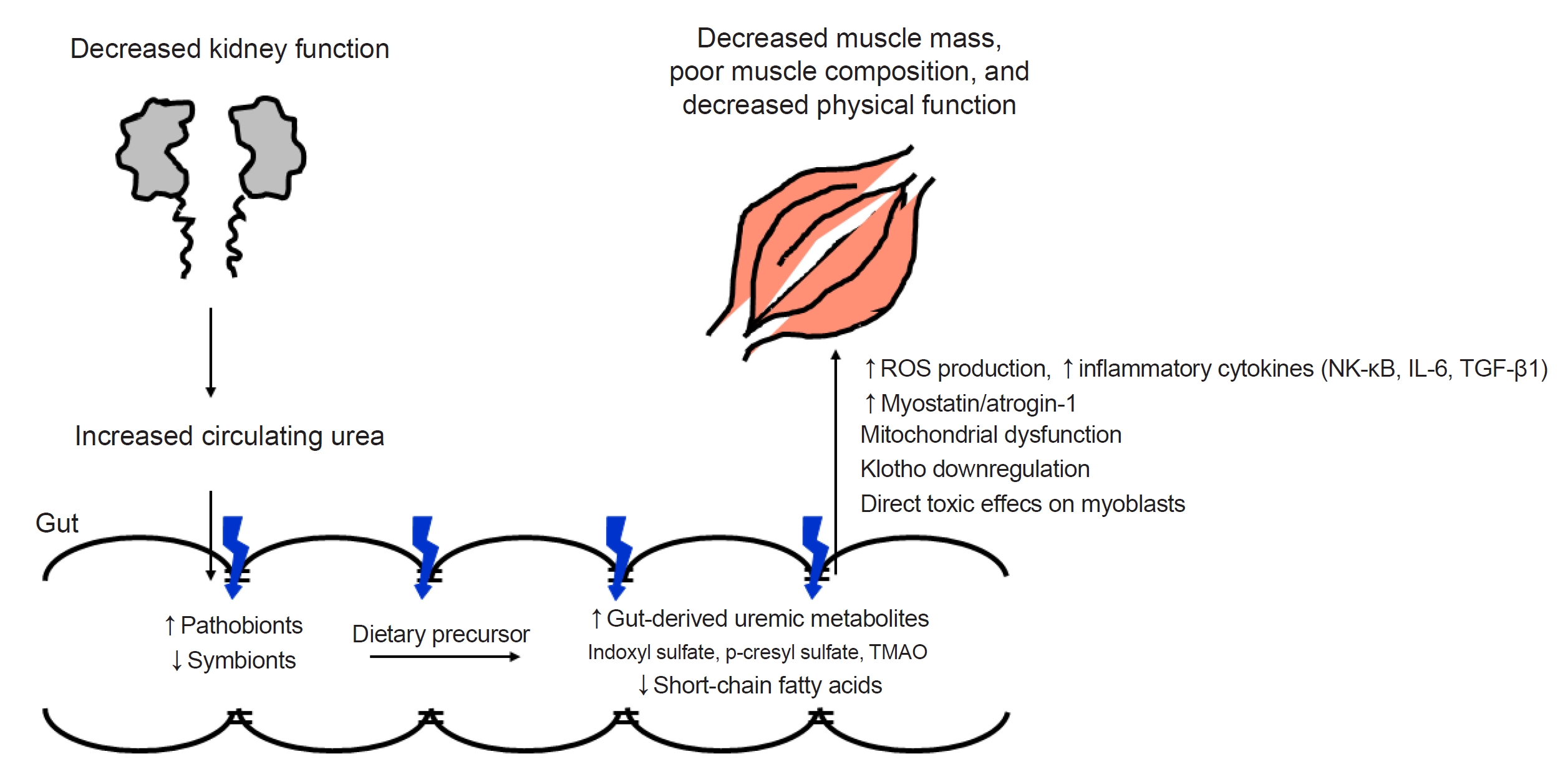
43. Lau WL, Savoj J, Nakata MB, Vaziri ND. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins.
Clin Sci (Lond) 2018;132:509–522.



44. Cigarran Guldris S, González Parra E, Cases Amenós A. Gut microbiota in chronic kidney disease.
Nefrologia 2017;37:9–19.


45. Al Khodor S, Shatat IF. Gut microbiome and kidney disease: a bidirectional relationship.
Pediatr Nephrol 2017;32:921–931.



47. Lustgarten MS. The kidney-gut-muscle axis in end-stage renal disease is similarly represented in older adults.
Nutrients 2019;12:106.



48. Niwa T, Yazawa T, Maeda K, et al. Effect of oral sorbent, AST-120, on serum concentration of indoxyl sulfate in uremic rats.
Nihon Jinzo Gakkai Shi 1990;32:695–701.

49. Niwa T. Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: experimental and clinical effects of oral sorbent AST-120.
Ther Apher Dial 2011;15:120–124.


50. Watanabe H, Miyamoto Y, Honda D, et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase.
Kidney Int 2013;83:582–592.


51. Deguchi T, Ohtsuki S, Otagiri M, et al. Major role of organic anion transporter 3 in the transport of indoxyl sulfate in the kidney.
Kidney Int 2002;61:1760–1768.


53. Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells.
Kidney Int 2003;63:1671–1680.

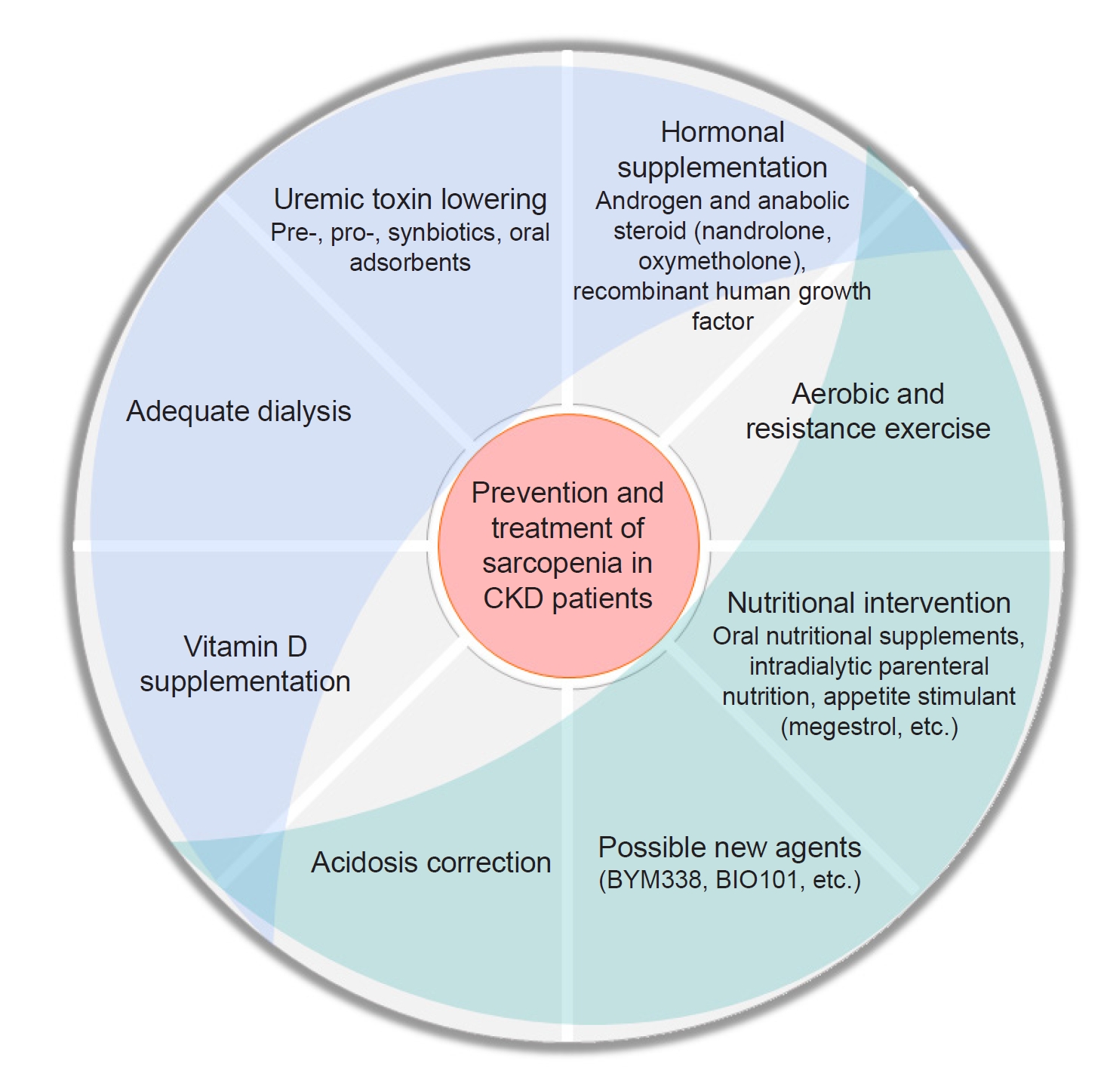
54. Sato E, Saigusa D, Mishima E, et al. Impact of the oral adsorbent AST-120 on organ-specific accumulation of uremic toxins: LC-MS/MS and MS imaging techniques.
Toxins (Basel) 2017;10:19.



56. Lin YL, Liu CH, Lai YH, et al. Association of serum indoxyl sulfate levels with skeletal muscle mass and strength in chronic hemodialysis patients: a 2-year longitudinal analysis.
Calcif Tissue Int 2020;107:257–265.



57. Shimizu H, Bolati D, Adijiang A, et al. Indoxyl sulfate downregulates renal expression of Klotho through production of ROS and activation of nuclear factor-ĸB.
Am J Nephrol 2011;33:319–324.



58. Avin KG, Coen PM, Huang W, et al. Skeletal muscle as a regulator of the longevity protein, Klotho.
Front Physiol 2014;5:189.



59. Semba RD, Ferrucci L, Sun K, et al. Low plasma klotho concentrations and decline of knee strength in older adults.
J Gerontol A Biol Sci Med Sci 2016;71:103–108.


60. Phelps M, Pettan-Brewer C, Ladiges W, Yablonka-Reuveni Z. Decline in muscle strength and running endurance in klotho deficient C57BL/6 mice.
Biogerontology 2013;14:729–739.



61. Rodrigues GG, Dellê H, Brito RB, et al. Indoxyl sulfate contributes to uremic sarcopenia by inducing apoptosis in myoblasts.
Arch Med Res 2020;51:21–29.


62. Takkavatakarn K, Wuttiputinun T, Phannajit J, Praditpornsilpa K, Eiam-Ong S, Susantitaphong P. Protein-bound uremic toxin lowering strategies in chronic kidney disease: a systematic review and meta-analysis.
J Nephrol 2021;34:1805–1817.



63. Kim JT, Kim SH, Min HK, et al. Effect of dialysis on aryl hydrocarbon receptor transactivating activity in patients with chronic kidney disease.
Yonsei Med J 2020;61:56–63.



65. Garito T, Zakaria M, Papanicolaou DA, et al. Effects of bimagrumab, an activin receptor type II inhibitor, on pituitary neurohormonal axes.
Clin Endocrinol (Oxf) 2018;88:908–919.

66. Heymsfield SB, Coleman LA, Miller R, et al. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial.
JAMA Netw Open 2021;4:e2033457.



67. Dioh W, Tourette C, Del Signore S, et al. A Phase 1 study for safety and pharmacokinetics of BIO101 (20-hydroxyecdysone) in healthy young and older adults.
J Cachexia Sarcopenia Muscle 2023;14:1259–1273.



69. Stenvinkel P, Carrero JJ, von Walden F, Ikizler TA, Nader GA. Muscle wasting in end-stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies.
Nephrol Dial Transplant 2016;31:1070–1077.


70. Fernández-Lucas M, Elías S, Ruiz-Roso G, Díaz M, Teruel-Briones JL, Quereda C. Treatment with megestrol acetate increases muscle mass in uraemic patients.
Nefrologia 2013;33:425–426.

71. Smith CS, Logomarsino JV. Using megestrol acetate to ameliorate protein-energy wasting in chronic kidney disease.
J Ren Care 2016;42:53–59.



72. Wazny LD, Nadurak S, Orsulak C, Giles-Smith L, Tangri N. The efficacy and safety of megestrol acetate in protein-energy wasting due to chronic kidney disease: a systematic review.
J Ren Nutr 2016;26:168–176.


73. Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial.
JAMA 1999;281:1275–1281.


74. Macdonald JH, Marcora SM, Jibani MM, Kumwenda MJ, Ahmed W, Lemmey AB. Nandrolone decanoate as anabolic therapy in chronic kidney disease: a randomized phase II dose-finding study.
Nephron Clin Pract 2007;106:c125–c135.



75. Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial.
J Am Soc Nephrol 2006;17:2307–2314.

76. Supasyndh O, Satirapoj B, Aramwit P, et al. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients.
Clin J Am Soc Nephrol 2013;8:271–279.


77. Pupim LB, Flakoll PJ, Yu C, Ikizler TA. Recombinant human growth hormone improves muscle amino acid uptake and whole-body protein metabolism in chronic hemodialysis patients.
Am J Clin Nutr 2005;82:1235–1243.


78. Kopple JD, Brunori G, Leiserowitz M, Fouque D. Growth hormone induces anabolism in malnourished maintenance haemodialysis patients.
Nephrol Dial Transplant 2005;20:952–958.


79. Ikizler TA, Wingard RL, Breyer JA, Schulman G, Parker RA, Hakim RM. Short-term effects of recombinant human growth hormone in CAPD patients.
Kidney Int 1994;46:1178–1183.


80. Ikizler TA, Wingard RL, Flakoll PJ, Schulman G, Parker RA, Hakim RM. Effects of recombinant human growth hormone on plasma and dialysate amino acid profiles in CAPD patients.
Kidney Int 1996;50:229–234.


81. Feldt-Rasmussen B, Lange M, Sulowicz W, et al. Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk.
J Am Soc Nephrol 2007;18:2161–2171.


82. Johannsson G, Bengtsson BA, Ahlmén J. Double-blind, placebo-controlled study of growth hormone treatment in elderly patients undergoing chronic hemodialysis: anabolic effect and functional improvement.
Am J Kidney Dis 1999;33:709–717.


83. Liu H, Bravata DM, Olkin I, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly.
Ann Intern Med 2007;146:104–115.


84. Taskapan H, Baysal O, Karahan D, Durmus B, Altay Z, Ulutas O. Vitamin D and muscle strength, functional ability and balance in peritoneal dialysis patients with vitamin D deficiency.
Clin Nephrol 2011;76:110–116.


85. Mori A, Nishino T, Obata Y, et al. The effect of active vitamin D administration on muscle mass in hemodialysis patients.
Clin Drug Investig 2013;33:837–846.



86. Singer R, Chacko B, Talaulikar G, Karpe K, Walters G. Placebo-controlled, randomized clinical trial of high-dose cholecalciferol in renal dialysis patients: effect on muscle strength and quality of life.
Clin Kidney J 2018;12:281–287.



87. Hewitt NA, O’Connor AA, O’Shaughnessy DV, Elder GJ. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients.
Clin J Am Soc Nephrol 2013;8:1143–1149.



88. de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status.
J Am Soc Nephrol 2009;20:2075–2084.



89. Stein A, Moorhouse J, Iles-Smith H, et al. Role of an improvement in acid-base status and nutrition in CAPD patients.
Kidney Int 1997;52:1089–1095.


90. Williams AJ, Dittmer ID, McArley A, Clarke J. High bicarbonate dialysate in haemodialysis patients: effects on acidosis and nutritional status.
Nephrol Dial Transplant 1997;12:2633–2637.


91. van Krimpen SJ, Jansen FA, Ottenheim VL, Belzer C, van der Ende M, van Norren K. The effects of pro-, pre-, and synbiotics on muscle wasting, a systematic review-gut permeability as potential treatment target.
Nutrients 2021;13:1115.



93. Tominaga K, Tsuchiya A, Nakano O, et al. Increase in muscle mass associated with the prebiotic effects of 1-kestose in super-elderly patients with sarcopenia.
Biosci Microbiota Food Health 2021;40:150–155.



94. Rossi M, Johnson DW, Morrison M, et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial.
Clin J Am Soc Nephrol 2016;11:223–231.


95. Choi E, Yang J, Ji GE, et al. The effect of probiotic supplementation on systemic inflammation in dialysis patients.
Kidney Res Clin Pract 2022;41:89–101.



96. Chen C, Wang J, Li J, Zhang W, Ou S. Probiotics, prebiotics, and synbiotics for patients on dialysis: a systematic review and meta-analysis of randomized controlled trials.
J Ren Nutr 2023;33:126–139.


97. Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH. Effects of synbiotics and probiotics supplementation on serum levels of endotoxin, heat shock protein 70 antibodies and inflammatory markers in hemodialysis patients: a randomized double-blinded controlled trial.
Probiotics Antimicrob Proteins 2020;12:144–151.



98. Lopes RC, Theodoro JM, da Silva BP, et al. Synbiotic meal decreases uremic toxins in hemodialysis individuals: a placebo-controlled trial.
Food Res Int 2019;116:241–248.


99. Kikuchi M, Ueno M, Itoh Y, Suda W, Hattori M. Uremic toxin-producing gut microbiota in rats with chronic kidney disease.
Nephron 2017;135:51–60.



100. Vaziri ND, Yuan J, Khazaeli M, Masuda Y, Ichii H, Liu S. Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption.
Am J Nephrol 2013;37:518–525.



102. Adijiang A, Niwa T. An oral sorbent, AST-120, increases Klotho expression and inhibits cell senescence in the kidney of uremic rats.
Am J Nephrol 2010;31:160–164.



103. Cha RH, Kang SH, Han MY, An WS, Kim SH, Kim JC. Effects of AST-120 on muscle health and quality of life in chronic kidney disease patients: results of RECOVERY study.
J Cachexia Sarcopenia Muscle 2022;13:397–408.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















