| Kidney Res Clin Pract > Volume 42(1); 2023 > Article |
|
Abstract
Notes
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) number 200162/2020-9 and by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) number E-26/202.524/2019.
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: LSGM, ISCB, DCMVR, LT, TRC, ME, LFMFC, PS, DM
Funding acquisition, Methodology: DM
Supervision: PS, DM, PGS
Writing–original draft: LSGM, ISCB, DCMVR, LT, TRC, ME, LFMFC, DM
Writing–review & editing: LSGM, LFMFC, PS, PGS, DM
All authors read and approved the final manuscript.
Acknowledgments
Figure 1.
Antioxidant and anti-inflammatory actions of cinnamon in cells.
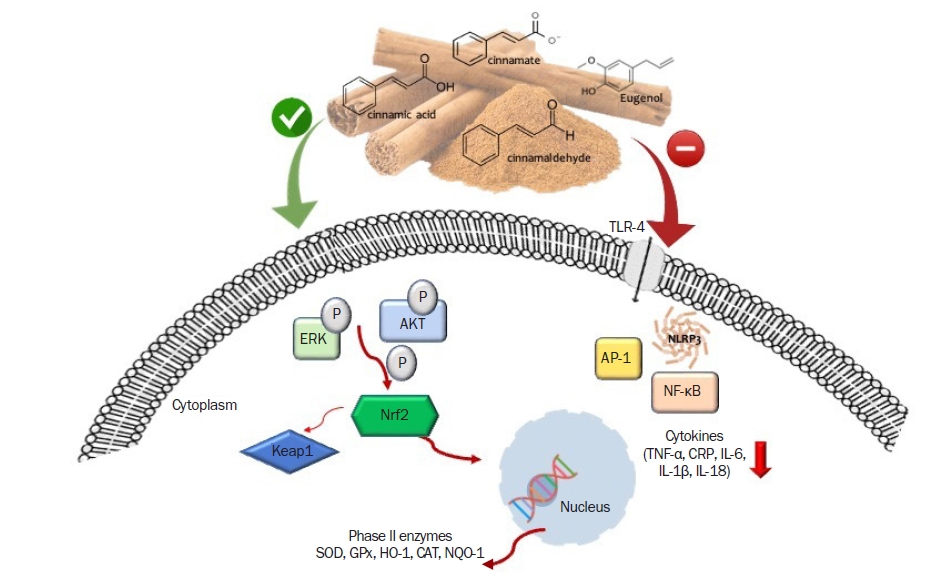
Figure 2.
The potential benefits of cinnamon to patients with diabetes, obesity, or CVDs.

Table 1.
Table 2.
| Reference | Study/samples | Intervention | Results |
|---|---|---|---|
| In vitro study | |||
| Uchi et al. (2017) [36] | Human keratinocyte cell line benzo[a]pyrene-stimulated | Cinnamaldehyde (25 μM) or Cinnamomum cassia extract (100 mg/mL) | ↑ Nrf2 translocation and HO-1 expression |
| ↓ activation of AHR | |||
| Kim et al. (2018) [35] | Raw 264.7 murine macrophage cells | Trans-cinnamaldehyde (25, 50, or 100 μM) | ↓ TNF-α, IL-1β, and IL-6 and NO synthesis |
| LPS-induced | |||
| Schink et al. (2018) [37] | THP-1 monocyte-macrophage cell line TIB-202, LPS-stimulated | Cinnamon compounds (25 μg/mL) | Trans-cinnamaldehyde and p-cymene ↓ IL-8 secretion |
| Qu et al. (2019) [38] | LPS-stimulated RAW264.7 cells | Cinnamaldehyde (5, 10, or 20 μM) pretreatment | ↓ NLRP3 inflammasome, miR-21 and miR-155 |
| ↓ ROS, the phosphorylation of AKT, mTOR, and COX-2 protein level | |||
| Cheng et al. (2020) [39] | Human rheumatoid fibroblast-like synoviocyte line MH7A cells IL-1β-induced | Cinnamaldehyde (40, 60, and 80 nM) pretreatment | 40, 60, and 80 nM: ↓ TNF-α, IL-6 |
| Chen et al. (2020) [33] | Human osteoarthritis chondrocytes LPS-induced | Cinnamaldehyde pretreatment (10, 20, or 50-μM) | All doses: ↓ IL-6, IL-1β, TNF-α |
| ↓ MMP-13 and ADAMTS-5 | |||
| Doses of 20 and 50 μM: LPS-stimulated NF-κB expression | |||
| Ben Lagha et al. (2021) [40] | The monoblastic leukemia cell line U937 LPS-stimulated | Cinnamon bark aqueous extract (32.5 to 500 μg/mL) pretreatment | 250 μg/mL: ↓ IL-6, IL-8, and TNF-α |
| Vallion et al. (2022) [41] | Human keratinocytes cells | 100 μM of cinnamaldehyde | ↑ Nrf2 accumulation |
| ↓ IL-1β transcription | |||
| Chen et al. (2022) [42] | LPS-induced human osteoarthritis synovial fibroblasts | Pretreatment with cinnamic aldehyde (20 and 50 μmol/L) | ↓ IL‐1β, IL‐6, and TNF‐α |
| ↓ TLR-4 and MyD88 expression | |||
| Experimental study | |||
| Tuzcu et al. (2017) [9] | HFD rats | Cinnamon polyphenol (100 mg/kg body weight) for 12 weeks | ↓ NF-κB p65 expressions |
| ↑ PPAR-α, IRS-1, Nrf2, and HO-1 expressions in the HFD rat livers | |||
| Abou El-Ezz et al. (2018) [43] | LPS-induced neuroinflammation mouse model | Trans-cinnamaldehyde (50 mg/kg) intraperitoneally for 1 week | ↓ IL-1β levels, MDA, and caspase-3 levels in the hippocampus |
| Activate Nrf2 | |||
| ↑ Glutathione S-transferase | |||
| Liu et al. (2020) [44] | in vitro: macrophages (Raw246.7) LPS-induced | In vitro: cinnamaldehyde (6.25, 12.5, or 25 μM) | In vitro: ↓ IL-1β, NLRP3 (12.5, and 25 μM) |
| In vivo: arthritis rat model, complete Freund’s adjuvant-induced | In vivo: cinnamaldehyde (200 mg/kg) orally for 4 weeks | ↓ TNF-α and NO (6.25, 12.5, and 25 μM) | |
| In vivo: ↓ IL-1 β in blood | |||
| ↓ NLRP3 in synovium | |||
| Wang et al. (2020) [45] | Leptin receptor-deficient (db/db) mice | Diet containing 0.02% cinnamaldehyde for 12 weeks | ↓ ROS generation, preserved NO production |
| ↑ p-eNOS | |||
| ↑ Nrf2, HO-1 and NQO-1 | |||
| Ryu et al. (2020) [46] | Mice with cognitive dysfunction induced by d-galactose and aluminum chloride | Trans-cinnamaldehyde (30 mg/kg/day) injected intraperitoneally + treadmill exercise for 5 weeks | ↑ Nrf2, NQO-1, HO-1, and SOD-1 |
| Abdel-kawi et al. (2022) [47] | Wistar rats, gastric ulcers ethanol-induced model | 2.5 mL/kg of cinnamon oil and omeprazole (20 mg/kg) for 1 week before ulcer induction | ↑ CAT, SOD, GPx, and GSH in the stomach |
| ↓ MDA and TNF-α levels | |||
| Zou et al. (2022) [34] | Sepsis-induced C57BL/6 J mice | 2 g/kg of cinnamyl alcohol by gavage | ↓ IL-1β and IL-18 |
| ↓ Expression of NLRP3, caspase-1, and apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain in the liver, heart, lungs, and kidneys | |||
ADAMTS-5, metalloproteinase with thrombospondin motif 5; AHR, aryl hydrocarbon receptor; AKT, protein kinase B; CAT, catalase; COX-2, cyclooxygenase type 2; GPx, glutathione peroxidase; GSH, glutathione; HFD, high-fat diet; HO-1, heme oxygenase 1; IL, interleukin; IRS-1, insulin receptor substrate 1; LPS, lipopolysaccharide; MDA, malondialdehyde; MMP-13, matrix metalloproteinase-13; mTOR, mammalian target of rapamycin; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa B; NLRP3, NLR family pyrin domain containing 3; NO, nitric oxide; NQO-1, NAD(P)H dehydrogenase [quinone] 1; Nrf2, nuclear factor erythroid 2-related factor 2; p-eNOS, phosphorylated endothelial nitric oxide synthase; PPAR-α, peroxisome proliferator-activated receptors (PPAR) alpha; ROS, reactive oxygen species; SOD-1, superoxide dismutase 1; TLR-4, toll-like receptor 4; TNF-α, tumor necrosis factor alpha.
Table 3.
| Reference | Study/sample | Intervention | Results |
|---|---|---|---|
| Experimental study | |||
| Hafizur et al. (2015) [58] | STZ-induced diabetic rats | 5 and 10 mg/kg of cinnamic acid or cinnamaldehyde | Cinnamic acid: ↓ blood glucose, improved glucose tolerance |
| ↑ Glucose-stimulated insulin secretion in isolated islets. | |||
| Cinnamaldehyde: ↔ glucose-stimulated insulin secretion | |||
| Qusti et al. (2016) [59] | STZ-induced diabetic in male albino rats | 20% (w/w) cinnamon methanol extract for 28 days | ↓ Blood glucose |
| ↓ IL-6 and MDA | |||
| ↑ CAT and SOD | |||
| ↓ Urea, Cr, and uric acid | |||
| Jawale et al. (2016) [60] | STZ-induced diabetic in rats | 10, 20, or 40 mg/kg of cinnamaldehyde for 3 weeks | ↓ Blood glucose |
| ↓ TNF-α and IL-6 | |||
| Hosni et al. (2017) [61] | STZ-induced diabetic in female albino rats with gestational diabetes | 20 mg/kg oral dose of cinnamaldehyde with or without fatty-sucrose diet, or normal diet for 8 weeks | ↓ Hyperphagia and glucose intolerance |
| ↓ Fructosamine, TC, TG, leptin | |||
| ↓ TNF-α, MDA, NO | |||
| ↑ HDL-C, adiponectin, liver glycogen | |||
| ↑ PPAR-γ gene expression | |||
| Taheri et al. (2018) [62] | STZ-induced diabetic in adult male Wistar rats | 300 mg/kg cinnamon bark powder for 14 days | ↓ CYP2D |
| Abdelmageed et al. (2019) [55] | STZ-induced T2D in male rats | 10 mg/kg of cinnamaldehyde for 2 months | ↓ OGTT, ITT, FBG |
| ↓ Insulin and HOMA-IR | |||
| ↑ HOMA-β | |||
| ↓ MDA | |||
| ↑ Aortic GSH, SOD, IRS-1, PI3K-p85, AKT2 | |||
| Kommula et al. (2020) [63] | Neonatal STZ rat model | 3% Cinnamon for 8 months | ↓ Fasting and postprandial glucose levels prevented retinal functional abnormalities |
| Mohammed et al. (2020) [64] | STZ-induced diabetic rats | 200 and 400 mg/kg of cinnamon oil emulsion in whey protein concentrate for 1 month | ↓ Blood glucose, amylase, |
| ↓ TC, LDL-C, TG | |||
| ↑ Insulin, HDL-C | |||
| ↑ Hepatic SOD, GSH | |||
| ↓ Hepatic MDA | |||
| Niazmand et al. (2021) [65] | STZ-induced diabetic rats | Cinnamon extract (100, 200, 400 mg/kg) and metformin (300 mg/kg) orally for 42 days | ↓ MDA level, SOD and CAT activities in the liver and kidney |
| Sampath et al. (2021) [66] | Gastric emptying in obesity-induced diabetic female mice | Cinnamaldehyde 50 mg per body mass per day for 6 weeks | ↓ Body weight gain |
| ↓ FBG | |||
| ↓ HOMA-IR | |||
| ↑ Reduced/oxidized glutathione ratio | |||
| Vijayakumar et al. (2022) [57] | STZ-induced diabetic rats | Ethanolic bark extracts of Cinnamomum cassia with different concentrations (300, 400, and 500 mg/kg BW) and glibenclamide (3 mg/kg BW) | ↑ Activities of mitochondrial enzymes |
| ↓ Levels of hepatic marker enzymes (AST, ALT, and ALP) | |||
| ↓ Urea, Cr, and uric acid | |||
| Çelik et al. (2022) [67] | STZ-induced diabetic rats | 20 mg/kg of BW of cinnamaldehyde by gavage daily for 1 month | ↓ FBG |
| ↓ TG, TC, VLDL, LDL-C, and urea levels | |||
| Human study | |||
| Bernardo et al. (2015) [68] | Nondiabetic adults | 100 mL of cinnamon tea (Cinnamomum burmannii bark) obtained from 60 g sticks of cinnamon soaked into 1,000 mL of water, after OGTT | Slightly ↓ PBG level after OGTT |
| Sengsuk et al. (2015) [69] | T2D patients | 1,500 mg of cinnamon (divided into 3 times a day capsules) or placebo for 2 months | ↓ Median glucose, TG, TG/HDL-C ratio, and BP |
| ↑ HDL-C and eGFR | |||
| Anderson et al. (2015) [71] | Hyperglycemic adults | 1 g (divided into 2 capsules) a day of water extract of cinnamon (CinSulin), or placebo for 2 months | ↓ FBG, HOMA-IR |
| ↓ Serum glucose 2 hours after 75 g carbohydrate load | |||
| ↓ Fructosamine, fasting insulin | |||
| ↓ TC, LDL-C, HDL-C | |||
| Azimi et al. (2016) [56] | T2D patients | 3 g/day of cinnamon with black tea for 2 months | ↓ ICAM-1 |
| ↔ BP and endothelial function | |||
| Gutierrez et al. (2016) [70] | Young, sedentary, obese women | 5 g of encapsulated cassia cinnamon bark for 3 separate days (30-, 60-, 90-, and 120-minute following glucose ingestion) | ↔ Insulin resistance and sensitivity |
| ↓ Peak blood glucose at 30-time point | |||
| Gupta Jain et al. (2017) [72] | Individuals with metabolic syndrome | 3 g (divided into 6 capsules) of cinnamon or placebo, for 4 months | ↓ FBG, ↓ HbA1c |
| ↓ WC, ↓ BMI improved lipid profile, waist-hip ratio, and BP | |||
| Talaei et al. (2017) [73] | T2D patients | 3 g of cinnamon (divided into 3 capsules-day), for 2 months | ↔ FBG, insulin, HbA1c, HOMA-IR, carboxymethyl lysine, total antioxidant capacity, and MDA |
| Zare et al. (2019) [74] | T2D patients | 1 g of cinnamon bark powder (divided into 2 capsules daily) or placebo for 3 months | ↓ BMI, body fat, visceral fat |
| ↓ FBG, HbA1c, fasting insulin, and insulin resistance | |||
| ↓ TC, LDL-C, and HDL-C | |||
| Kizilaslan and Erdem (2019) [75] | Healthy adult individuals | 1 g or 3 g or 6 g/day cinnamon peel (C. cassia), for 40 days | ↔ BMI, HbA1c |
| Difference in pre-prandial blood glucose (6 g/day) | |||
| Difference in postprandial blood glucose on days 20 and 40 for 1, 3, and 6 g of cinnamon | |||
| Davari et al. (2020) [51] | T2D patients | 3 g of cinnamon for 2 months | ↔ NF-κB, SIRT1, hs-CRP, IL-6, and TNF-α plasma levels |
| Romeo et al. (2020) [76] | Adults with prediabetes | 500 mg cinnamon thrice daily for 3 months | Fasting plasma glucose remained stable only in the cinnamon group |
| ↓ OGTT | |||
| Lira Neto et al. (2022) [77] | T2D patients | 3 g of cinnamon (capsules daily) for 3 months | ↓ HbA1c |
| ↓ Fasting venous glucose | |||
| Rachid et al. (2022) [78] | T2D patients | 6 g/100 mL of aqueous cinnamon extract (C. burmannii) after 30, 60, 90, and 120 minutes | ↔ Area under the curve, glucose conc., variation, and maximum glucose conc |
AKT, protein kinase B; AKT2, AKT serine/threonine kinase 2; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; BW, body weight; CAT, catalase; Cr, creatinine; CYP2D, cytochrome P450; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; GSH, glutathione; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HOMA-β, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment-estimated insulin resistance; hs-CRP, high-sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; IRS-1, insulin receptor substrate 1; ITT, insulin tolerance test; LDL-C, low-density lipoprotein cholesterol; MDA, malondialdehyde; NF-κB, nuclear factor kappa B; NO, nitric oxide; OGTT, oral glucose tolerance test; PBG, postprandial glucose level; PI3K, phosphoinositide 3-kinase ; PPAR-γ, peroxisome proliferated activated receptor gamma; SIRT 1, silent mating-type information regulation 2 homolog 1; SOD, superoxide dismutase; STZ, streptozotocin; T2D, type 2 diabetes; TC, total cholesterol; TG, triglyceride; TNF-α, tumor necrosis factor alpha; VLDL, very-low-density lipoprotein; WC, waist circumference.
Table 4.
| Reference | Study/sample | Intervention | Results |
|---|---|---|---|
| Experimental study | |||
| Lopes et al. (2015) [111] | Adult male Wistar rat | 400 mg/kg BW/day of cinnamon aqueous extract (Cinnamomum zeylanicum), for 25 days | ↔ Food intake and serum lipid profile |
| ↓ Body mass gain | |||
| ↓ Relative mass of WAT | |||
| Leptin mRNA expression in the WAT | |||
| ↑ Protein content | |||
| Lee et al. (2016) [112] | 3T3-L1 preadipocytes cells | 50, 100, 200 μg/mL of cinnamon extract (Cinnamomum cassia) | ↑ Lipid storage in white adipocytes, |
| ↑ Fatty acid oxidation capacity | |||
| ↑ PGC-1α, CPT-1α, PPARγ, C/EBP-α, and C/EBP-β genes expressions | |||
| Khare et al. (2016) [113] | 3T3-L1 preadipocytes cells | 10, 20, and 40 μM of cinnamaldehyde: in vitro | ↑ HPL |
| In vivo: male Swiss albino mice | 5 mL/kg and 10 mL/kg BW of cinnamaldehyde with a normal or HFD: in vivo | ↓ Expression of perilipin and GPD | |
| ↓ PPARγ and C/EBP-α prevented the increase in visceral fat pad weight regulated leptin/ghrelin ratio | |||
| ↑ Anorectic gene expression in hypothalamus (POMC, BDNF, UCN, CARTPT, and CCK) | |||
| ↓ Glycerol and free fatty acid levels | |||
| ↑ Expression levels of lipolysis-promoting genes: HSL, PNPLA2, and MGLL | |||
| ↓ IL-1β, COX, MCP1, TNF-α, and IL-6 | |||
| ↑ Anorectic and lipolytic gene expression | |||
| Jiang et al. (2017) [52] | Primary preadipocytes from and human adipose-derived stem cells | 200 and 400 μM of cinnamaldehyde | ↑ Thermogenesis: ↑ UCP1, FGF21, PKA, phosphorylation of HSL and PLIN1 |
| ↑ Lipid metabolism: Pdk4 | |||
| Kwan et al. (2017) [114] | 3T3-L1 preadipocytes and | 80 µg/mL (in vitro) and 500 mg/kg BW (in vivo) cinnamon extract (C. cassia) | Induced browning in white adipocytes: ↑ UCP1 expression; ↑ Prdm16, Cidea, PPARγ, PGC, Cpt1 |
| Ex vivo: subcutaneous adipose tissue from db/db mice and in vivo/ex vivo DIO mice | Induced browning in subcutaneous adipocytes in db/db mice: UCP1 protein and mRNA Cidea and Prdm16 | ||
| DIO mice: ↑ UCP1 expression in the subcutaneous adipose tissue; ↓ BW | |||
| Kang et al. (2019) [115] | 3T3-L1 and HIB1B preadipocytes cells | 10–200 μM of trans-cinnamic acid of bark (C. cassia) | Induced browning in white adipocytes activation of β3AR-PKA-AMPK, TRPA1, and GPR signaling pathways |
| ↑ Fat oxidation | |||
| ↓ Adipogenesis and lipogenesis | |||
| Neto et al. (2019) [116] | Lactating dams (Wistar rats) were supplemented, and adult male offspring were evaluated at 180 days old | 400 mg/kg BW/day of cinnamon aqueous extract (C. zeylanicum) during lactating period | ↑ Visceral obesity |
| Hepatic metabolic dysfunction and ↑ lipid accumulation | |||
| ↓ Glycogen content in the liver, hyperleptinemia and hyperinsulinemia | |||
| Neto et al. (2020) [117] | Adolescent rat model of obesity programmed by early overnutrition | Cinnamaldehyde 40 mg/kg of body mass per day for 29 days | ↓ Visceral adipose tissue mass |
| Ataie et al. (2021) [118] | Adult male Wistar rats with HFD-induced | Cinnamaldehyde 20 mg/kg of body mass per day for 16 weeks | ↓ Plasma nitrate and nitrate |
| ↓ Islet insulin secretion | |||
| ↓ iNOS activity | |||
| Li et al. (2022) [105] | Adult male Wistar rat obesity HFD-induced | Cinnamon powder 50 or 100 mg/kg BW orally for 12 weeks | ↓ Hepatic levels of oxidative and inflammatory biomarkers |
| ↓ Serum levels of glucose, liver enzymes, insulin, and lipid profiles | |||
| ↓ Hepatic expression of SREBP-1c and NF-κB | |||
| ↑ PPAR-α, CD36, CPT-1, and Nrf-2 | |||
| Neto et al. (2022) [119] | Adolescent rat model of obesity programmed by early overnutrition | Cinnamaldehyde 40 mg per kg of body mass per day for 30 days | ↓ Adipocyte hypertrophy |
| ↑ Oxidative pathways (PGC1α, FGF21) in WAT | |||
| ↑ Increased BAT thermogenesis markers (PPARα, FGF21, UCP-1) | |||
| ↓ WAT adipocyte size | |||
| Miah et al. (2022) [120] | Adult Swiss albino mice hyperlipidemia and obesity | 10% butter with cinnamon 200 mg, 400 mg, or 600 mg powder per liter drinking water for 10 weeks | ↓ TC, LDL-C, and glucose levels |
| Butter enriched HFD-induced | ↓ ALT and AST and fat deposition in the liver | ||
| Human study | |||
| Gupta Jain et al. (2017) [72] | Adults with metabolic syndrome | 3 g/day (6 capsules) of cinnamon for 16 weeks | ↓ BW, WC, waist-to-hip ratio |
| ↓ % Body fat | |||
| Borzoei et al. (2017) [121] | Polycystic ovary syndrome in overweight or obese women | 1.5 g cinnamon extract (3 capsules) for 8 weeks | Improved glucose metabolism and lipid profile, ↓ insulin |
| Khedr et al. (2020) [110] | Overweight /obese adults | 1.2 g of Ceylon cinnamon capsules and 120 mg of Orlistat for 15 weeks | ↓ BMI |
| ↓ Lipase activity | |||
| ↓ Lipid profile | |||
| Wang et al. (2021) [122] | Normal and overweight/obese individuals | 1/2 cup dry instant oatmeal with milk prepared with or without 6 g of cinnamon (Korintje cinnamon, from cassia bark), acute intake (4 hours) | ↓ Postprandial insulin response in overweight/obese individuals |
| ↓ Postprandial glucagon levels, glucagon and C-peptide response in normal weight participants | |||
| Huang et al. (2022) [123] | Overweight adults | 6 g of cinnamon meal on 4 separate visits at least 3 days apart | ↓ Postprandial glycemia |
ALT, alanine aminotransferase; AMPK, adenosine monophosphate-activated protein kinase; AST, aspartate aminotransferase; BAT, brown adipose tissue; BDNF, brain-derived neurotrophic factor; BMI, body mass index; BW, body weight; C/EBP-α, CCAAT/enhancer-binding protein alpha; C/EBP-β, CCAAT-enhancer-binding protein beta; CARTPT, cocaine amphetamine-related transcript; CCK, cholecystokinin; CD36, cluster of differentiation 36; Cidea, DFFA-like effector A; COX, cyclooxygenase; CPT-1, carnitine palmitoyl transferase 1; CPT-1α, carnitine palmitoyltransferase 1 alpha; DIO, diet-induced obesity; FGF21, fibroblast growth factor 21; GPD, glycerol-3-phosphate dehydrogenase; GPR, G-protein-coupled receptor; HFD, high-fat diet; HSL, hormone-sensitive lipase; IL, interleukin; iNOS, inducible nitric oxide synthase; LDL-C, low-density lipoprotein cholesterol; MCP-1, monocytechemotactic protein 1; MGLL, monoglyceride lipase; NF-κB, factor nuclear kappa B; Nrf-2, nuclear factor erythroid 2-related factor 2; Pdk4, pyruvate dehydrogenase kinase 4; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1 alpha; PKA, protein kinase A; PLIN1, lipid droplet-associated protein perilipin 1; PNPLA2, patatin phospholipase domain containing 2; POMC, proopiomelanocortin; PPARα, peroxisome proliferator-activated receptor alpha; PPARγ, peroxisome proliferator-activated receptor gamma; Prdm16, PR domain containing 16; SREBP-1, sterol regulatory element-binding transcription factor 1; TC, total cholesterol; TNF-α, tumor necrosis factor alpha; TRPA1, necrosis factor receptor-associated protein 1; UCN, urocortin; UCP-1, uncoupling protein 1; WAT, white adipose tissue; WC, waist circumference; β3AR, β3 adrenergic receptor.
Table 5.
| Reference | Study/sample | Intervention | Results |
|---|---|---|---|
| Experimental study | |||
| Kwon et al. (2015) [131] | Rat aortic vascular smooth muscle cells | Extract Cinnamomum cassia bark – 10, 30, and 50 µM | ↓ PLCγ1, Akt, and P38 |
| ↑ Percentage of G0/G1 phase cells | |||
| ↓ PCNA expression | |||
| Panickar et al. (2015) [132] | Mouse brain endothelial cells | Cinnamtannin D1 – 10−2 and 10−3 mg/mL | ↓ OGD-induced swelling |
| ↓ Cell swelling in presence of MCP-1 | |||
| ↓ Mitochondrial ROS | |||
| ↓ OGD-induced fluorescence | |||
| Chen et al. (2016) [133] | Mice with ischemia/reperfusion-induced brain injury | 10, 20, and 30 mg/kg trans-cinnamaldehyde, an essential oil in cinnamon powder 60 minutes before ischemia surgery | ↓ Infarction area and neurological deficit score |
| ↓ iNOS, COX-2, NF-κB, mRNA, TNF-α | |||
| Kang et al. (2016) [134] | Male rats with metabolic syndrome with cardiac oxidative stress | 20, 40, and 80 mg/kg cinnamaldehyde for 5 weeks | ↓ HW/BW, TGF-β, p-Smad 2/3 and Smad4 |
| ↑ GSH/GSSG | |||
| Tuzcu et al. (2017) [9] | Rats given high-fat feed | 100 mg/kg cinnamon polyphenol extract for 12 weeks | ↓ Expression of hepatic SREBP-1c, LXRs, ACLY, FAS, MDA, NF-κB |
| ↑ PPAR-α, IRS, Nrf2, HO-1, SOD, CAT | |||
| ↓ TG, TC, LDL-C | |||
| ↓ BW, visceral fat | |||
| Nayak et al. (2017) [135] | Mice with dexamethasone-induced atherosclerosis | 500 mg/kg and 250 mg/kg cinnamon extract for 12 days | ↓ TG, TC, LDL-C |
| ↑ HDL-C | |||
| ↓ Atherosclerotic change of aorta | |||
| Sedighi et al. (2018) [136] | Rats with ischemia | Cinnamomum zeylanicum bark extract – 50, 100, or 200 mg/kg – 2 weeks before ischemia | ↓ Infarct size |
| ↓ Ventricular tachycardia, ventricular ectopic beats episodes | |||
| ↓ R-wave amplitude | |||
| ↑ Heart rate during ischemia | |||
| ↓ MDA, cardiactroponin I, LDH | |||
| ↑ SOD, GPx | |||
| Pulungan and Pane (2020) [137] | Mice (Mus musculus) given high-fat feed | 2, 4, and 8 mg/kg cinnamon extract for 2 weeks | ↓ TC |
| Alsoodeeri et al. (2020) [138] | Rats given high-fat feed | 2 and 4 g/kg cinnamon powder for 4 weeks | ↓ TG, TC, LDL-C |
| ↑ HDL-C | |||
| Wang et al. (2020) [45] | Leptin receptor-deficient mice | Diet containing 0.02% cinnamaldehyde for 12 weeks | ↑ Nitrotyrosine, NO, NRF2, HO-1, NQO-1 |
| ↓ ROS, p-eNOS | |||
| Moreno et al. (2022) [139] | Rings from male Wistar rat thoracic aorta pre | Cinnamon extract (0–380 μg/mL) | Induced concentration-dependent vasodilation |
| Tian et al. (2022) [140] | Male, cardiac hypertrophy model C57BL/6 | Trans-cinnamaldehyde daily at a dosage of 50 mg/kg or 100 mg/kg via oral gavage for 2 weeks | Inhibited induced cardiac hypertrophy |
| Human study | |||
| Ranasinghe et al. (2017) [141] | Healthy adults | 85 mg, 250 mg, and 500 mg of C. Zeylanicum (water extract) for a period of 3 months, with dose increased at monthly intervals | SBP, DBP |
| ↔Renal and liver function, fasting blood glucose, HDL-C, VLDL, and TG | |||
| ↓TC and LDL-C | |||
| Mirmiran et al. (2019) [142] | Type 2 diabetes patients | 3 g cinnamon extract capsules, for 2 months | ↓ICAM-1 and VCAM-1 in both cinnamon and placebo groups, but not between groups |
| Shirzad et al. (2021) [143] | Stage 1 hypertension patients | Cinnamon capsules, 1,500 mg/day, for 2 months | Moderate clinical decrease in mean ambulatory SBP |
| ↑ HDL-C | |||
| ↓ LDL-C levels | |||
| Zhang et al. (2022) [144] | Patients with mild stroke or transient ischemic attack | Aspirin-cinnamon group (100 mg/day aspirin + 5 g of cinnamon granules) and aspirin-placebo group (100 mg/day aspirin + placebo granules) for 2 months | Aspirin-cinnamon group: |
| ↓TG, LDL-C, fasting plasma glucose, HbA1c, Lp-PLA2, and hs-CRP | |||
| ↑ HDL-C | |||
| ↓ Carotid atherosclerosis |
ACLY, ATP-citrate lyase; Akt, protein kinase B; BW, body weight; CAT, catalase; COX-2, cyclooxygenase type 2; DBP, diastolic blood pressure; FAS, fatty acid synthase; GPx, glutathione peroxidase; GSH/GSSG, glutathione/oxidized glutathione ratio; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HO-1, heme oxygenase 1; hs-CRP, high-sensitivity C-reactive protein; HW/BW, heart-to-body weight; ICAM-1, intercellular adhesion molecule 1; iNOS, inducible nitric oxide synthetase; IRS, insulin receptor; LDH, lactate dehydrogenase; LDL-C, low-density lipoprotein cholesterol; Lp-PLA2, plasma lipoprotein-related phospholipase A2; LXRs, liver X receptor; MCP-1, monocyte chemoattractant protein 1; MDA, malondialdehyde; mRNA, messenger RNA; NF-κB, nuclear factor kappa B; NO, nitric oxide; NQO-1, NAD(P)H dehydrogenase [quinone] 1; NRF2, factor erythroid nuclear factor 2 related to factor 2; OGD, oxygen-glucose deprivation; P38, anti-phospho-p38; PCNA, antiproliferating cell nuclear antigen; p-eNOS, phosphorylated endothelial nitric oxide synthase; PLCγ1, anti-phospho-phospholipase C gamma 1; PPAR-α, peroxisomeproliferator-activated receptor alpha; p-Smad 2/3, phosphorylated Smad2/3; p-Smad4, phosphorylated Smad4; ROS, reactive oxygen species; SBP, systolic blood pressure; SOD, superoxide dismutase; SREBP-1c, sterol regulatory element-binding proteins; TC, total cholesterol; TG, triglyceride; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha; VCAM-1, vascular cell adhesion molecule-1; VLDL, very-low-density lipoprotein.
Table 6.
| Reference | Study/sample | Intervention | Results |
|---|---|---|---|
| Hussain et al. (2019) [170] | Administration of acetaminophen in BALB/c mice | Pretreatment with 200 mg/kg/day i.g. of cinnamon bark aqueous extract for 2 weeks | Prevention against elevation in serum ALT, AST, Cr, urea |
| Prevention against macroscopic and histological alterations in liver and kidney | |||
| Improvement of oxidative balance | |||
| Niazmand et al. (2021) [65] | STZ-induced diabetic rats | 100, 200, or 400 mg/kg of cinnamon extract for 6 weeks | ↓ MDA level, SOD and CAT activities in the liver and kidney |
| ↑ GSH and total thiol contents and NO production | |||
| Alshahrani et al. (2021) [171] | Male Wistar rats with nephrotoxicity induced by acetaminophen | 50, 100, and 200 mg/kg of cinnamon oil with 2 g/kg of acetaminophen, for 15 days | Improvement in serum biochemical markers and oxidative parameters: |
| Protected cellular injury in kidney tissue | |||
| ↓ IL-1β, IL-6, and caspase 3 and 9 | |||
| ↑ GSH level and ameliorates antioxidative enzymes (SOD, CAT, GR, and GPx in kidney tissue) | |||
| Atsamo et al. (2021) [172] | Male Wistar rats with gentamicin-induced nephrotoxicity | 200 and 400 mg/kg/day of Cinnamomum zeylanicum stem bark aqueous extract for 2 weeks concomitantly with gentamicin administration | Prevention of alterations in body weight, serum total proteins, calcium level, kidneys’ relative weight, Cr, urea, and uric acid |
| ↓ MDA and TNF-α, IL-1β, and IL-6 and nitrites | |||
| ↑ GSH, SOD, CAT | |||
| Prevention of histological alterations | |||
| Elshopakey and Elazab, (2021) [173] | Broiler chickens with copper-induced nephrotoxicity | 200 mg/kg of C. zeylanicum alone or plus probiotic for 6 weeks | Both supplementations: |
| ↓ Urea, Cr, and uric acid | |||
| In renal tissue: | |||
| ↓ MDA, ↑CAT, and GSH, ↓ Copper | |||
| ↓ TNF-α, IL-2, Bax, and COX-II in kidneys | |||
| ↑ IL-10 and Bcl-2 | |||
| Xiao (2022) [168] | Sprague-Dawley rats (male) kidney senescence model D-galactose-induced | 40 mg/kg/day of cinnamaldehyde for 6 weeks | ↓ Blood urea nitrogen and Cr |
| In the kidneys: the contours of the proximal and distal convoluted tubules were improved, ↓ the number of nuclear pyknosis, ↓ hyperemia | |||
| ↑ Ratio of p-P13K to P13K and the ratio of p-Akt to Akt |
Akt, protein kinase B; ALT, alanine transaminase; AST, aspartate transaminase; Bcl-2, B-cell lymphoma 2; CAT, catalase; COX-II, cyclooxygenase; Cr, creatinine; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; IL, interleukin; MDA, malondialdehyde; NO, nitric oxide; p21, p21/WAF1Cip1; PARP, poly (ADP-ribose) polymerase; PI3K, phosphoinositide 3-kinase; SOD, superoxide dismutase; TNF-α, tumor necrosis factor alpha.
References
-
METRICS

- ORCID iDs
-
Laís de Souza Gouveia Moreira

https://orcid.org/0000-0003-0576-1418Isabela de Souza da Costa Brum

https://orcid.org/0000-0002-1392-403XDrielly C. M. de Vargas Reis

https://orcid.org/0000-0002-5250-7220Liana Trugilho

https://orcid.org/0000-0002-3282-4755Tuany R. Chermut

https://orcid.org/0000-0001-8907-4134Marta Esgalhado

https://orcid.org/0000-0002-0370-5175Ludmila F. M. F. Cardozo

https://orcid.org/0000-0001-8507-2369Peter Stenvinkel

https://orcid.org/0000-0002-8785-4820Paul G. Shiels

https://orcid.org/0000-0002-7577-9843Denise Mafra

https://orcid.org/0000-0001-6752-6056 - Related articles
-
Pharmacologic therapeutics in sarcopenia with chronic kidney disease2024 March;43(2)
Urinary podocyte markers in diabetic kidney disease
Novel biomarkers for diabetic kidney disease2022 September;41(Suppl 2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















