Introduction
Chronic kidney disease (CKD), which affects more than 10% of the global population, is often irreversible and progresses to kidney failure. Although the prevalence of CKD in children is much lower than that in adults, the disease has a significant clinical influence during childhood. Children with CKD have different comorbidities than adults, seizure disorders, neurocognitive delays, and congenital heart disease, that confound their care and contribute to a heavy burden of care coordination [
1]. In addition, children and adolescents with CKD face lifelong increases in morbidity and mortality and decreased quality of life [
2]. However, because most information is adult-focused, there is a suboptimal understanding of pediatric CKD.
Current CKD guidelines suggest a stage-based approach to standardize the assessment of CKD severity and management of this disease [
3]. Patients with CKD are classified based on their level of kidney function or estimated glomerular filtration rates (eGFRs), however, CKD staging does not consider the change in kidney function over time. Clinicians usually consider the trajectory of eGFR for a patient because it can impact treatment decisions [
4]. In 1976, Mitch et al. [
5] first reported that patients with CKD showed a linear decline in reciprocal serum creatinine concentration over time. To date, much of the literature on kidney function decline assumes a linear pattern of progression. More recently, it has become apparent that patients with similar CKD stages can follow different trajectories [
6,
7] and that the rate of decline in eGFR in CKD may vary even among those with similar diagnoses [
6,
8–
11]. Thus, understanding kidney function trajectory may be difficult because some patients experience nonlinear disease progression, while others may exhibit stable kidney function over many years [
12,
13]. Clinicians usually consider the trajectory of eGFR for a patient because it may impact treatment decisions such as the frequency of follow-up and preparation for kidney replacement therapy [
4]. A review of patterns of kidney function trajectory and factors that influence this trajectory in adult CKD has been published. In the adult CKD population, eGFR trajectory is important as a predictor of various clinical outcomes such as the risk of kidney failure, cardiovascular disease, and mortality in patients with non–dialysis-dependent CKD. Although CKD is a major health problem in both adults and children, the disease is far from the same in these two populations [
14]. The etiology and progression rate of as well as treatment strategies for pediatric CKD are different from those in adults [
15,
16]. Few studies have prospectively evaluated the progression of CKD in children and adolescents with factors associated with progression such as proteinuria, hypoalbuminemia, hypertension (HTN), dyslipidemia, and anemia [
17]. The decline in kidney function is not always linear in children, and the rate of decline in kidney function varies regardless of the baseline GFR, similar to that in adults [
9,
12,
16]. Therefore, the aims of this study are as follows: i) to identify different patterns of subsequent decline in kidney function; and ii) to investigate factors associated with different patterns of eGFR trajectories in pediatric CKD.
Discussion
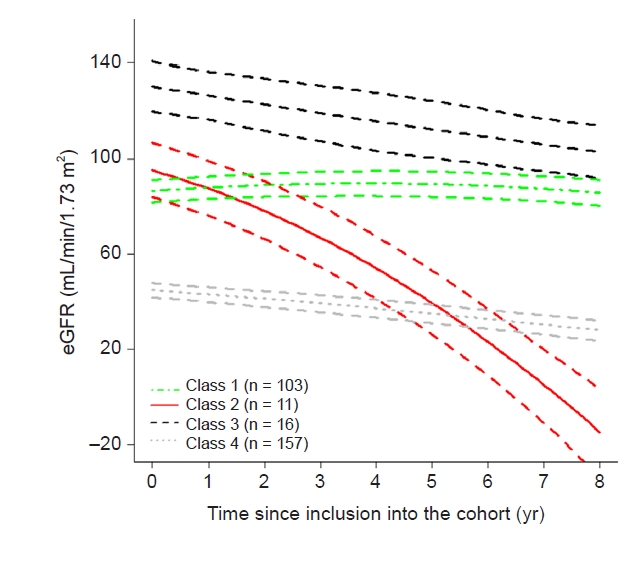
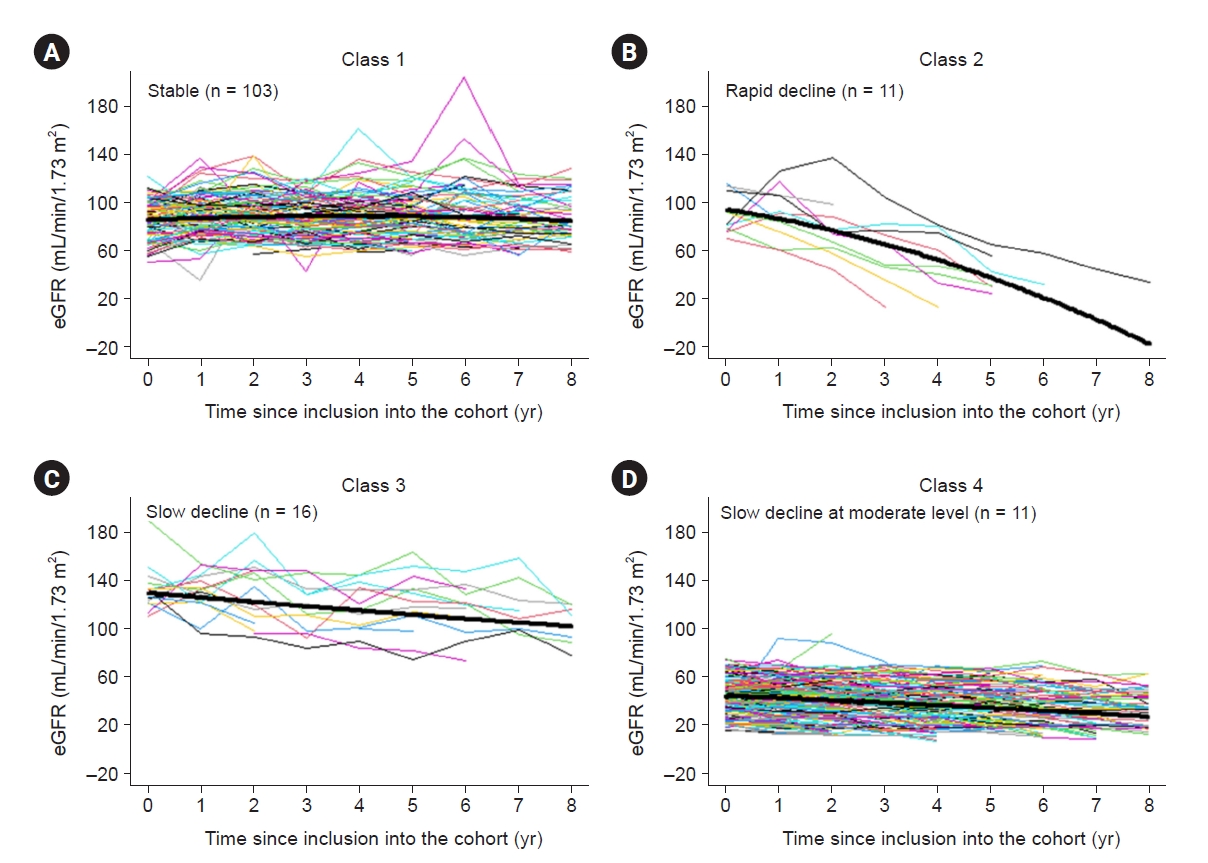
In this study, we investigated the progression of pediatric CKD and eGFR trajectory patterns by modeling using an LCMM. We found that those with a near normal eGFR at baseline (classes 1–3) did not show a uniform trajectory; some did not progress (class 1), while others progressed rapidly (class 2) or slowly (class 3). In addition to class 4, which had a lower baseline eGFR, approximately two-thirds of subjects showed a decline in eGFR irrespective of their baseline eGFRs (classes 2–4); however, in the rest, deterioration of kidney function was not apparent over the follow-up period (class 1). The eGFR trajectories were linear in the vast majority of the study population of children with CKD, whereas 5.6% of subjects showed a strong nonlinear eGFR decline, similar to the findings of previous studies in adults (class 2) [
9,
10].
The natural course of CKD progression is known to vary according to the underlying conditions in children [
12,
33]. To define GFR decline in pediatric CKD, we divided the two groups by the type of primary kidney disease. However, simply grouping patients according to the original disease of glomerulopathy vs. non-glomerulopathy (mainly CAKUT) did not discriminate the pattern of progression well, with the annual decline in eGFR of 2.3 mL/min/1.73 m
2 in glomerular disease vs. 1.7 mL/min/1.73 m
2 in non-glomerular disease (p > 0.05). This finding is different from that of the CKiD cohort in which children with glomerular disease were found to progress more quickly to kidney failure than children with non-glomerular disease [
34]. These differences may stem from differences in the inclusion criteria between the CKiD and KNOW-Ped CKD studies, in which stage 1 CKD was included. While the subjects in the CKiD study had a median eGFR of 44 mL/min/1.73 m
2 and an annual decline in eGFR of 1.8 mL/min/1.73 m
2, the study population analyzed in this study had a median eGFR of 63.3 mL/min/1.73 m
2 at enrollment, and the annual decline in eGFR was 1.5 mL/min/1.73 m
2. Although the patients are small, the patients with CKD stage 3 or higher showed no difference in the annual decline in eGFR according to the original disease in our cohort. The majority of patients with stage 1 CKD had glomerular disease, and the median eGFR of patients with glomerular disease in our cohort was higher than those observed in the CKiD study [
35], the North American Pediatric Renal Transplant Cooperative Study [
36], and the India study [
37]; this may explain why the decline in kidney function was not significantly more rapid in the glomerulopathy group than in the non-glomerulopathy group in this study.
The management of CKD focuses on delaying or preventing its progression, thus understanding CKD progression is important. Existing guidelines for CKD suggest a stage-based approach [
3]. To date, the pattern of kidney function trajectory seems to be more important for understanding and managing CKD. It has become clear that, even in patients with stage 4 or 5 CKD, a significant portion of these patients may have stable renal function [
4]. Patients with a slower trajectory may require less intensive follow-up. In this study, the differences between classes 1 (stable eGFR) and 2 (rapid decline in eGFR) included the proportion of patients with glomerulopathy as the cause of CKD and the uPCR (low in class 1). After adjusting for age, baseline eGFR, cause of CKD, hyperuricemia, and proteinuria, only nephrotic-range proteinuria was significantly associated with a rapid decline. This finding is similar to the findings of Jungers et al. [
35], who found that the degree of proteinuria was one of the major determinants of CKD progression in adults. Therefore, children with CKD with non-nephrotic proteinuria may have a stable trajectory of eGFR and thus require less frequent follow-up. On the other hand, those with nephrotic-range proteinuria are expected to progress rapidly, as previously shown, and may need more frequent follow-up visits [
35]. The prevalence of anemia, dyslipidemia, and HTN was also not different between the rapid progressors (class 2) and stable group (class 1), while in the CKiD study, nephrotic-range proteinuria, hypoalbuminemia, HTN, dyslipidemia, male sex, and anemia were associated with a significantly more rapid decline [
10,
18]. Since the majority of classes 1 and 2 were patients with early stages of CKD (stages 1 and 2) at enrollment and half of them had CKD stage 1, factors that are previously known to be related to a rapid GFR decline other than proteinuria might not be significant in patients with stage 1 CKD. In class 3, the median eGFR was above normal at enrollment and moderate decline over time, reaching a normal range. After adjusting for age, baseline eGFR, cause of CKD, hyperuricemia, and proteinuria, only baseline eGFR was associated between classes 1 (stable eGFR) and 3 (moderate decline in eGFR). An eGFR that is higher than normal may suggest glomerular hyperfiltration, a known risk factor for accelerated renal function decline in diabetic populations [
38]; however, its relevance in nondiabetic populations remains yet to be identified. Previously reported equations such as the kidney failure risk equation and CKiD calculator to determine the risk of progression to kidney failure in children with CKD have been validated only for those with moderately impaired kidney function [
39,
40]. However, these equations do not include CKD with normal kidney function, thus, estimating the time to progression to kidney failure in children with CKD with a normal or high GFR is difficult. Since the small numbers of patients, further study is needed to determine exactly. As expected, those with a decreased eGFR at baseline experienced progression (class 4). However, it was more stable than class 2 or 3, which had lower proportions of patients with CAKUT than class 4, in which approximately 60% of patients had CAKUT. The annual eGFR decline of class 4 was faster, with an annual decline in eGFR of 2.4 mL/min/1.73 m
2 than that of the general non-glomerulopathy group of 1.52 mL/min/1.73 m
2 in our study, that of 1.3 mL/min/1.73 m
2 per year in the CKiD study [
28], and that of 1.0 mL/min/1.73 m
2 per year in those with congenital kidney disease [
40]. In class 4, anemia, hyperuricemia, metabolic acidosis, and hyperuricemia were evident; these are characteristics of patients with a lower eGFR than those in class 1. After adjusting for age, baseline eGFR, cause of CKD, hyperuricemia, and proteinuria, only baseline eGFR was associated between classes 1 and 4.
Kidney disease trajectories have been traditionally assessed by plotting the slope of 1/creatinine. Several studies have recently applied more sophisticated modeling techniques including LCMMs instead of simple linear models [
9,
41]. An LCMM is a method developed to identify classes of individual longitudinal trajectories of quantitative indicators [
10]. An LCMM supposes that the patterns of a population in a specific state are diverse and consist of a number of latent classes of subjects identified by estimated mean profiles of trajectories. This method has been used to evaluate various medical conditions, such as depression [
42], atherosclerosis [
43], or disability [
44], to identify clinical meaning and suggest relevant guidance. In kidney disease, an LCMM was used to identify the distinct kidney function trajectories in CKD patients [
22]. In this study, the proportions of patients of the four classes were diverse. Two of them represented only 3.8% and 5.6% of the population, however, a similar pattern was observed when the groups were divided differently. The proportions of each kidney trajectory class are available in
Supplementary Table 1 (available online). Most of the patients (10 of 11) who were classified as class 2 were assigned to the same class even when divided into three or five groups. In class 3, all patients were assigned to class 3 when divided into three groups, and approximately half of the patients were assigned to class 3 when divided into five groups. Even if the number of groups differed, classes 2 and 3 showed a unique pattern; thus, the data are not scattered and are included in the same group. Therefore we assumed that the application of an LCMM could identify smaller subgroups of the population with pediatric CKD. Our study is the first report to reveal heterogeneity in the kidney function trajectory using an LCMM in pediatric patients with stage 1 to 4 CKD and to demonstrate that kidney function trajectories were not completely determined by the CKD stage at enrollment or the cause of CKD. Such modeling is valid to draw meaningful conclusions since few subjects reached the endpoint of kidney failure because our study included a significant number of subjects with stage 1 CKD (n = 64).
Our study has some strengths and limitations. First, half of the subjects had early CKD (CKD stages 1 and 2); therefore, information regarding advanced CKD might be insufficient. On the other hand, this may be a strength of this study. We identified changes in eGFR in early CKD, and even though the participants had CKD stages 1 and 2, the average decline of –1.5 per year is still considerable. Second, the prevalence of HTN, a known risk factor for CKD progression was similar at enrollment in each group. It is worth noting that most rapid progressors were already receiving treatment with RAAS inhibitors, which may have influenced the study results. Furthermore, the investigation of new-onset HTN during the follow-up period was not within the scope of this study. Further research is warranted to better understand the clinical implications of HTN within this cohort. Third, despite the presence of numerous potential risk factors for disease progression among children with CKD, our study only identified nephrotic-range proteinuria as being associated with a rapid decline in eGFR. Given the relatively small sample size, it was not feasible to conduct a subgroup analysis for comorbidities associated with CKD and to investigate other factors such as low birth weight, prematurity, and medication use. Additionally, due to resource limitations, we were unable to assess the impact of acute kidney injury on the course of CKD. Fourth, although an LCMM is a powerful technique to elucidate the structure underlying population heterogeneity, some subgroups are too small, potentially limiting statistical power and the generalizability of the results. We also excluded patients younger than 2 years of age because of different normal eGFRs and patients with less than 2 years of follow-up to minimize the risk of confounding bias. These exclusions may have caused selection bias. Consequently, these inherent limitations present challenges in assessing and drawing definitive conclusions regarding the diverse patterns of CKD progression in children. However, the data were obtained from the KNOW-Ped CKD study, which is a relatively large Asian prospective pediatric CKD cohort with a long-term follow-up period, which is unique and provides considerable information.
In this study, we revealed kidney function trajectories and their determinants using a prospective cohort with a 9-year follow-up period. We characterized four distinct eGFR trajectories among pediatric subjects with CKD: those with a stable eGFR, those with a consistent fast decline, those with a consistent gradual decline, and those with a consistent slow decline. Subjects in the fast-declining trajectory had nephrotic-range proteinuria. Although the eGFR is not low in these patients, those with nephrotic-range proteinuria are expected to progress rapidly and may need more frequent follow-up.

















 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















