| Kidney Res Clin Pract > Volume 32(2); 2013 > Article |
|
Abstract
Sphingomonas paucimobilis is an aerobic Gram-negative bacillus found in soil and water. Knowledge regarding the role of this infectious agent is limited because it is rarely isolated from human material. Furthermore, it is an unusual pathogen in cases of peritoneal dialysis (PD)-associated peritonitis. The clinical courses and outcomes of peritonitis caused by S. paucimobilis are variable. Whereas some patients were cured with appropriate antibiotic therapy, others required catheter removal. Cases of PD-associated peritonitis caused by S. paucimobilis have been reported worldwide, and there was a case report of coinfection with S. paucimobilis and Chryseobacterium indologenes in Korea. However, there has been no case caused by S. paucimobilis as a single pathogen. We report a case of PD-associated peritonitis due to S. paucimobilis in which the patient recovered after catheter removal.
Keywords
Peritoneal dialysis, Peritonitis, Sphingomonas paucimobilisContinuous ambulatory peritoneal dialysis (CAPD) is an important treatment option for patients who are at the end stage of renal disease. Although the rate of peritonitis has declined in recent years because of improvements in the CAPD technique, peritonitis remains the leading cause for discontinuation of CAPD [1].
Sphingomonas paucimobilis is frequently isolated from environmental sources such as soil and water, and it is found on hospital equipment such as ventilators, nebulizers, and humidifiers [2], [3]. Although S. paucimobilis plays an extremely limited role as an infectious agent [4], it is responsible for two types of human infections: community-acquired infection and nosocomial infection [5]. Cases of peritonitis by S. paucimobilis have been reported rarely. The clinical outcomes were diverse in previously reported peritoneal dialysis (PD)-associated peritonitis cases [2], [5], [6], [7]. Furthermore, definitive guidelines do not exist for treating S. paucimobilis.
Cases of PD-associated peritonitis caused by S. paucimobilis have been reported worldwide, and one case caused by coinfection with S. paucimobilis and Chryseobacterium indologenes was reported in Korea [8]. However, this is the first case report of peritonitis due to S. paucimobilis as a single pathogen in Korea. We describe a case of PD-associated peritonitis due to S. paucimobilis that was resolved after catheter removal.
A 63-year-old man who had been treated with CAPD for 6 years was admitted to our hospital because of a turbid peritoneal effluent accompanied by constant abdominal pain that began 2 days prior to his admission. The underlying cause of the patientŌĆÖs end-stage renal disease was diabetes mellitus and hypertension, and his dialysis treatment consisted of four exchanges of 2 L dialysate per day. He had been engaged in rice farming and lived in the countryside. He had been treated for peritonitis with Pseudomonas aeruginosa and Staphylococcus epidermidis 7 months previously.
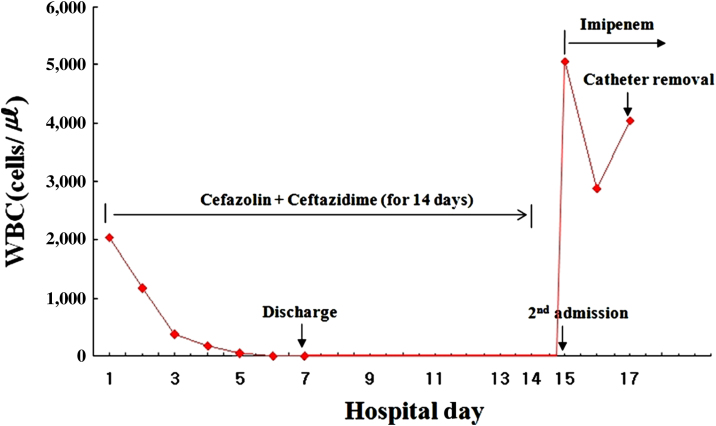
On admission, his blood pressure was 165/80 mmHg, heart rate was 84 beats/min, respiratory rate was 19/min, and body temperature was 36.8┬Ā┬░C. His abdomen was diffusely tender with normal bowel sounds, and infection was not found around the catheter exit site. The laboratory findings showed PD peritonitis: the white blood cell (WBC) count of the peritoneal effluent was 2,040/mm3 with a neutrophil predominance (85%). His hemoglobin was 11.3┬Āg/dL, WBC count was 7.6├Ś109/L, and C-reactive protein was 5.9┬Āmg/dL (normal: <0.8┬Āmg/dL). Bacteria were not observed on Gram stain examination. After peritoneal fluid was sent for bacterial culture, a single 1-g dose of cefazolin and a single 1-g dose of ceftazidime were given intraperitoneally per day. Two peritoneal fluid samples were inoculated into a BACTEC plus Aerobic/F culture bottle (Becton Dickinson Diagnostic Instrument System, Drogheda, Ireland) and incubated in a BACT/ALERT 3D Blood Culture System (Biomerieux, Marcy l├©toile, France). Culture of the peritoneal dialysate revealed S. paucimobilis, which was susceptible to ceftazidime, cefotaxime, imipenem, meropenem, gentamicin, ciprofloxacin, and minocycline, and resistant to piperacillin, aztreonam, and colistin.
The peritoneal WBC decreased to 30/mm3 and the patientŌĆÖs clinical condition improved on the fifth day after starting intraperitoneal ceftazidime. The patient was discharged on Day 7 with 7-day intraperitoneal antibiotics. The next day after 14 days of intraperitoneal ceftazidime, abdominal pain developed again, and the effluent became turbid. The peritoneal effluent contained 2,880 WBC/mm3, of which 75% were neutrophils. Abdominal computed tomography and ultrasonography were normal. We started a 1-g dose of intraperitoneal imipenem twice daily instead of cefazolin and ceftazidime. The culture of the peritoneal dialysate at second admission showed S. paucimobilis that was resistant to ceftazidime, ampicillin/sulbactam, piperacillin, cefotaxime, cefepime, aztreonam, and amikacin, and susceptible to imipenem, meropenem, ciprofloxacin, minocycline, and trimethoprim-sulfamethoxazole. However, the patientŌĆÖs abdominal pain persisted and the WBC count of the peritoneal effluents became elevated to 4,300/mm3 (Figure 1). Therefore, we removed the Tenckhoff catheter 3 days later despite continued imipenem therapy, and PD was switched to hemodialysis. The culture of the catheter tip was negative. Imipenem was continued for another week and the patientŌĆÖs clinical condition improved. The patient has been maintained on hemodialysis after catheter removal.
PD-associated peritonitis caused by S. paucimobilis is rare, and this bacillus has not been reported as the single causative pathogen of PD-associated peritonitis in Korea. We report a case of peritonitis due to S. paucimobilis that required catheter removal.
S. paucimobilis is a yellow-pigmented, gram-negative bacillus that has a single polar flagellum with slow mobility. These bacteria are aerobic, nonfermentative, oxidase positive, and catalase-positive [9]. Although human infections caused by S. paucimobilis are generally rare [4], these infections appear to have increased in humans in recent years [10]. Such a finding was probably considered to be associated with hospital-acquired infection due to indwelling intravascular devices [3], [10]. Peritonitis caused by S. paucimobilis has been reported in patients undergoing peritoneal dialysis. In Korea, only one case of peritonitis caused by S. paucimobilis and C. indologenes has been reported [8]. However, this is the first case report of PD-associated peritonitis due to S. paucimobilis as a single pathogen in Korea.
Since the first two cases of PD-associated peritonitis by S. paucimobilis were described by Glupczynski et al [2], several other cases have been reported, with diverse clinical courses and outcomes [2], [5], [6], [7]. Although Hsueh et al [3] suggested that imipenem alone or an aminoglycoside plus a third-generation cephalosporin could effectively treat infections caused by S. paucimobilis, no definitive guideline exists for the treatment of this organism. In previous reports, half of the reported cases were treated successfully with antibiotics alone, but the other half underwent catheter removal [2], [5], [6], [7]. In our case, S. paucimobilis continued to grow in peritoneal effluent cultures upon the patientŌĆÖs second admission, and the peritoneal WBC count was increased despite the use of antibiotics such as imipenem for 3 days. Therefore, we removed the Tenckhoff catheter and PD was switched to hemodialysis.
In the case discussed here, at first admission, the dialysate was clear and abdominal pain disappeared following intraperitoneal administration of ceftazidime, which was effective against S. paucimobilis. However, peritonitis by the same organism recurred on the 15th day during antibiotic treatment. In this circumstance, adding aminoglycoside or changing antibiotics to imipenem was helpful for treatment of peritonitis caused by S. paucimobilis
[3], so we decided to use imipenem because the patient had a urine output of more than 1,000┬ĀmL per day. According to International Society for Peritoneal Dialysis guidelines, antibiotics dose should be empirically increased by 25% in patients with residual renal function [11]. However, residual renal function was not considered in deciding the antibiotic dose. Thus, inadequate dosage of antibiotics might be one of the possible causes of treatment failures in this case.
In summary, we report a case of PD-associated peritonitis caused by S. paucimobilis that was treated by catheter removal. Therefore, catheter removal could be considered in cases of PD-associated peritonitis caused by S. paucimobilis. Adequate antibiotics dosage is essential to treat this rare peritonitis, especially in patients with residual renal function.
References
1. Troidle L, Gorban-Brennan N, Kliger A, Finkelstein F. Continuous peritoneal dialysis-associated peritonitis: A review and current concepts. Semin Dial 16:2003;428ŌĆō437.


2. Glupczynski Y, Hansen W, Dratwa M, Tielemans C, Wens R, Collart F, Yourassowsky E. Pseudomonas paucimobilis peritonitis in patients treated by peritoneal dialysis. J Clin Microbiol 20:1984;1225ŌĆō1226.



3. Hsueh PR, Teng LJ, Yang PC, Chen YC, Pan HJ, Ho SW, Luh KT. Nosocomial infections caused by Sphingomonas paucimobilis: Clinical features and microbiological characteristics. Clin Infect Dis 26:1998;676ŌĆō681.


4. Winn WC, Allen SD, Janda WM, Koneman EW, Schreckenberger PC. KonemanŌĆÖs color atlas and textbook of diagnostic microbiology. 6th ed.2006. Lippincott Williams & Wilkins; Philadelphia.

5. Dervisoqlu E, Meric M, Kalender B, Sengul E. Sphingomonas paucimobilis peritonitis: a case report and literature review. Perit Dial Int 28:2008;547ŌĆō550.


6. Reina J, Bassa A, Llompart I, Portela D, Borrell N. Infections with Pseudomonas paucimobilis: report of four cases and review. Rev Infect Dis 13:1991;1072ŌĆō1076.


7. Baddour LM, Kraus AP Jr, Smalley DL. Peritonitis due to Pseudomonas paucimobilis during ambulatory peritoneal dialysis. South Med J 78:1985;366


8. Yoon JS, Hwang EA, Chang MH, Park WY, Jin KB, Han SY, Park SB, Kim HC, Ryoo NH. Peritonitis by Chryseobacterium indologenes and Sphingomonas paucimobilis in a patient undergoing continuous ambulatory peritoneal dialysis (CAPD). Korean J Nephrol 20:2001;683ŌĆō694.
9. Acinetobacter von Graevenitz A.. Alcaligenes, Moraxella, and other non-fermentative gram-negative bacteria. In: Murray PR, Barron EJ, Pfaller MA, Tenover FC, Yolkens RH, Manual of clinical microbiology. 6th ed. 1995. American Society for Microbiology; Washington, DC: p. 520ŌĆō532.
10. Lin JN, Lai CH, Chen YH, Lin HL, Huang CK, Chen WF, Wang JL, Chung HC, Liang SH, Lin HH. Sphingomonas paucimobilis bacteremia in human: 16 case reports and a literature review. J Microbiol Immunol Infect 43:2010;35ŌĆō42.


11. Perit Dial Int.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















