| Kidney Res Clin Pract > Volume 34(3); 2015 > Article |
|
Abstract
Rothia muciliaginosa (R. mucilaginosa) is a facultative, Gram-positive coccus that is considered to be part of the normal flora of the mouth and respiratory tract. There are sporadic reports of the organism causing endocarditis in patients with heart valve abnormalities, as well as meningitis, septicemia, and pneumonia associated with intravenous drug abuse. However, it is an unusual pathogen in cases of peritoneal dialysis (PD)-associated peritonitis. Although R. mucilaginosa is generally susceptible to penicillin, ampicillin, cefotaxime, imipenem, rifampicin, and glycopeptides, there are no guidelines for the treatment of PD-associated peritonitis. Herein, we report a case of PD-associated peritonitis due to R. mucilaginosa that was resolved with intraperitoneal antibiotic treatment.
Keywords
Continuous ambulatory peritoneal dialysis, Peritonitis, Rothia mucilaginosaContinuous ambulatory peritoneal dialysis (CAPD) is an important treatment option for patients with end-stage renal disease. Although the rate of peritonitis has declined in recent years because of improvements in CAPD techniques, peritonitis remains the leading cause for discontinuation of CAPD [1].
Rothia muciliaginosa (R. mucilaginosa) is a component of the normal flora of the mouth and respiratory tract and is usually associated with dental plaques, cavities, and periodontal diseases [2,3]. It appears that the presence of an indwelling vascular catheter and previous treatment with ciprofloxacin increase the risk of invasive infection with Rothia
[4]. However, cases of peritonitis caused by R. mucilaginosa are rare. Furthermore, definitive guidelines do not exist for treating R. mucilaginosa–induced peritonitis.
Although cases of peritoneal dialysis (PD)-associated peritonitis caused by R. mucilaginosa have been reported worldwide, no Korean cases have been reported [5,6]. To the best of our knowledge, this is the first case report of peritonitis due to R. mucilaginosa as a single pathogen in Korea. Herein, we describe a case of PD-associated peritonitis caused by R. mucilaginosa that was cured after a 2-week course of intraperitoneal antibiotic therapy.
A 58-year-old Korean man, who had been treated with CAPD for 3 years, was admitted to our hospital because of turbid peritoneal effluent accompanied by constant abdominal pain that began 1 day before his admission. The underlying causes of his end-stage renal disease were diabetes mellitus and hypertension. He had two prior episodes of peritonitis in the past few years. He exchanged dialysis solutions of 2 L per dwell 4 times/d, with the solution remaining in the abdomen for 6 hours and had a urine output of about 200 mL/d.
On admission, his blood pressure was 120/80 mmHg, heart rate was 72 beats/min, respiratory rate was 18 breaths/min, and body temperature was 37.4°C. His abdomen was diffusely tender with normal bowel sounds, and infection was not found around the catheter exit site. The laboratory findings showed PD-associated peritonitis: the white blood cell (WBC) count of the peritoneal effluent was 1,620/mm3 with a neutrophil predominance (90%). His hemoglobin was 9.9 g/dL, WBC count was 14.5×109/L, and C-reactive protein was 12.53 mg/dL (normal: <0.8 mg/dL). Bacteria were not observed on Gram stain examination. After the peritoneal fluid was sent for bacterial culture, a single 1 g/d dose of cefazolin and a single 1 g/d dose of ceftazidime were given intraperitoneally. Two peritoneal fluid samples were inoculated into a BACTEC plus Aerobic/F culture bottle (Becton Dickinson Diagnostic Instrument System, Drogheda, Ireland) and incubated in a BacT/ALERT 3D Blood Culture System (Biomerieux, Marcy l`etoile, France). Culture of the peritoneal dialysate revealed Streptococcus mitis, which was susceptible to levofloxacin, clindamycin, vancomycin, and tetracycline.
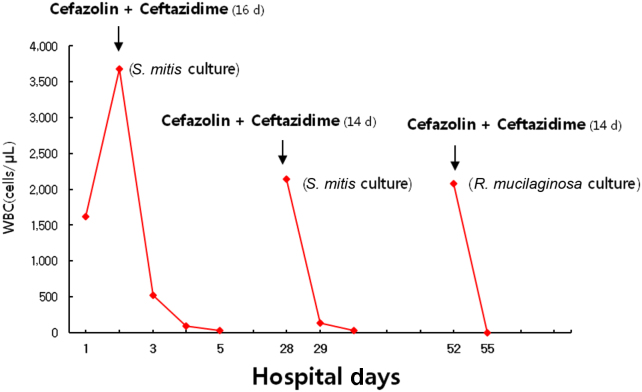
The peritoneal WBC count decreased to 30/mm3, and the patient׳s clinical condition improved on the 5th day after starting intraperitoneal cefazolin and ceftazidime. The patient recovered after a 2-week course of intraperitoneal antibiotics. However, he complained of abdominal pain and turbid peritoneal effluent 3 days after termination of intraperitoneal antibiotics. The WBC count of the peritoneal effluents was 2,140/mm3 with a neutrophil predominance (80%). We restarted intraperitoneal cefazolin and ceftazidime, and repeated culture of the peritoneal dialysate revealed S. mitis. According to the International Society for Peritoneal Dialysis guidelines, we recommended removal of the PD catheter, but he refused our recommendation. Therefore, we maintained intraperitoneal antibiotics, and the patient improved after intraperitoneal antibiotics for 2 weeks; however, the patient experienced turbid peritoneal effluent 10 days after cessation of the second round of intraperitoneal antibiotics. The WBC count of the peritoneal effluents was 2,080/mm3 with a neutrophil predominance (85%), and repeated culture of the peritoneal dialysate revealed R. mucilaginosa. The peritoneal WBC count decreased to 8/mm3, and abdominal pain disappeared on the 3rd day after starting intraperitoneal cefazolin and ceftazidime. The patient׳s condition improved, and he was discharged after a 2-week course of intraperitoneal antibiotics (Fig. 1).
During the admission period, the patient presented with a painful mass anteriorly over his left clavicle. X-ray showed a poorly defined, surface-based lesion arising from the proximal shaft of the left clavicle. Computed tomography scan revealed osteolytic bony destruction and soft tissue mass formation suggesting a malignant bone tumor, such as a chondrosarcoma or osteogenic sarcoma with pathologic fracture in the proximal portion of the left clavicle (Fig. 2). Although we recommended further workup and an operation, he refused.
PD-associated peritonitis caused by R. mucilaginosa is rare, and this coccus has not been reported as the single causative pathogen of PD-associated peritonitis in Korea. Here, we report a case of peritonitis due to R. mucilaginosa that recovered after intraperitoneal antibiotic therapy. A malignant bone tumor on the left clavicle was coincidentally found.
R. mucilaginosa is a Gram-positive, coagulase-negative, encapsulated, non–spore-forming coccus considered part of the commensal flora of the oral cavity and upper respiratory tract in humans. Infection in humans was first reported as endocarditis in 1978 [2,3,7]. R. mucilaginosa appears to be an opportunistic pathogen in immunocompromised patients and has been implicated in serious infections such as septicemia, endocarditis, meningitis, pneumonia, and osteomyelitis [4]. However, it is an unusual pathogen in cases of PD-associated peritonitis. Although cases of PD-associated peritonitis caused by R. mucilaginosa have been reported previously, there are no prior reports in Korea. To the best of our knowledge, this is the first case report of PD-associated peritonitis due to R. mucilaginosa in Korea.
The most common risk factors for Rothia infection are indwelling catheter, leukemia, cancer, cardiac valvular heart disease, intravenous drug abuse, severe neutropenia, and previous treatment with ciprofloxacin [8]. R. mucilaginosa is usually associated with the formation of dental plaque and tooth cavities. In our case, the patient did not have any recent history of dental work; however, he had a history of treatment with ciprofloxacin 2 months earlier and had a malignant bone tumor on the left clavicle. Therefore, this patient was at high risk for PD-associated peritonitis related to atypical organisms such as Rothia.
Recommendations for treatment of R. mucilaginosa-induced peritonitis were not included in the International Society for Peritoneal Dialysis guidelines [9]. However, based on previous literature, all patients with PD-associated peritonitis caused by R. mucilaginosa recovered after intraperitoneal antibiotic therapy including ampicillin, rifampin, vancomycin, and cefazolin [5,6,10]. It was reported that a patient with human immunodeficiency virus was successfully treated with intraperitoneal vancomycin [11]. Another patient who had recently lost a renal allograft and was still on immunosuppressive therapy developed coagulase-negative staphylococcal bacteremia and culture-negative peritonitis treated with ceftriaxone and ofloxacin with removal of PD catheter. Two months later, she was back on PD and had another episode of peritonitis, which grew R. mucilaginosa. It responded well to intraperitoneal vancomycin with resolution of symptoms in 2 days [12]. In our case, we did not perform an antibiotic sensitivity test because of the absence of Clinical Laboratory Standard Institute guidelines for this organism [6]. We empirically started intraperitoneal cefazolin and ceftazidime and maintained these antibiotics with clinical improvement. Such antibiotics could be helpful to treat R. mucilaginosa-induced peritonitis if sensitivity test results are not available.
In summary, we report a case of PD-associated peritonitis caused by R. mucilaginosa that was cured after a 2-week course of intraperitoneal antibiotic therapy. Intraperitoneal antibiotics should therefore be considered as treatment for PD-associated peritonitis caused by R. mucilaginosa.
References
1. Troidle L, Gorban-Brennan N, Kliger A, Finkelstein F. Continuous peritoneal dialysis-associated peritonitis: a review and current concepts. Semin Dial 16:2003;428–437.


2. Ruoff KL. Miscellaneous catalase-negative, gram-positive cocci: emerging opportunist. J Clin Microbiol 40:2002;1129–1133.



3. Bergan T, Kocur M. Stomatococcus mucilaginosus gen. nov., sp. nov., ep. rev., a member of the family Micrococcaceae. Int J Sys Bacteriol 32:1982;374–377.

4. McWhinney PM. Stomatococcus mucilaginosus: an emerging pathogen in neutropenic patients. Clin Infect Dis 14:1992;641–646.


5. Hodzic E, Synder S. A case of peritonitis due to Rothia mucilaginosa. Perit Dial Int 30:2010;379–383.



6. Gosmonova EO, Garrett FT, Wall BM. Peritonitis caused by Rothia mucilaginosa in a peritoneal dialysis patient. Am J Med Sci 346:2013;517–518.


7. Rubin SJ, Lyons RW. Murcia AJ: Endocarditis associated with cardiac catheterization due to a gram-positive coccus designated Micrococcus mucilaginosus incertae sedis. J Clin Microbiol 7:1978;546–549.



8. Ascher DP, Zbick C, White C, Fischer GW. Infections due to Stomatococcus mucilaginosus: 10 cases and review. Rev Infect Dis 13:1991;1048–1052.


9. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, Johnson DW, Kuijper EJ, Lye WC, Salzer W, Schaefer F, Struijk DG. International Society for Peritoneal Dialysis: Peritoneal dialysis-related infections recommendation: 2010 update. Perit Dial Int 30:2010;393–423.


10. Hayat A, Thaneeru P. Rothia mucilaginosa: a rare cause of peritoneal dialysis-related peritonitis. N Z Med J 18:2013;118–120.
Figure 1
Serial changes of white blood cell count in peritoneal effluents during hospitalization. Peritoneal white blood cell count increased up to 3,680/mm3 and recovered to normal levels in a short time span. R. mucilaginosa, Rothia mucilaginosa; S. mitis, Streptococcus mitis; WBC, white blood cell.
- TOOLS
-
METRICS

- Related articles
-
Acute interstitial nephritis induced by
Solanum nigrum 2016 December;35(4)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















