| Kidney Res Clin Pract > Epub ahead of print |
Abstract
Background
Methods
Results
Notes
Figure 1.
Kaplan-Meier survival curves according to the presence of intradialytic hypotension (IDH).
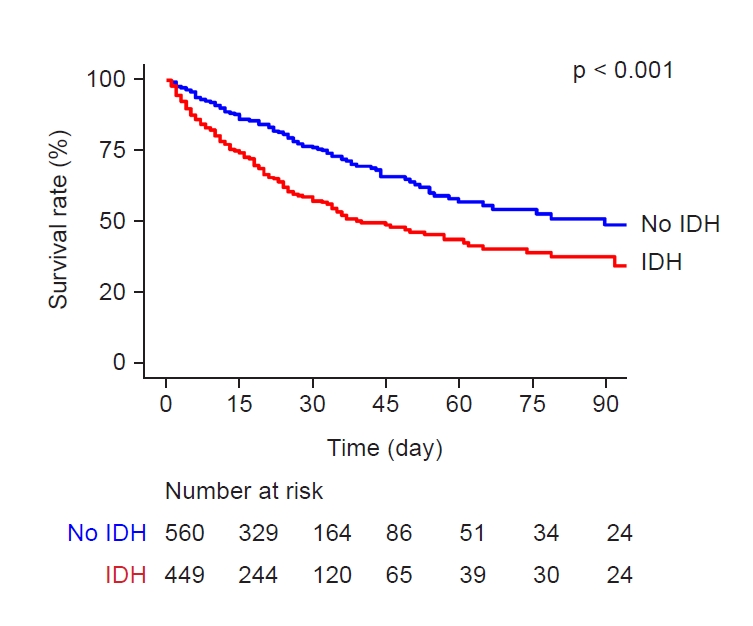
Figure 2.
Kaplan-Meier curves of the risk of transfer to the intensive care unit (ICU) according to the presence of intradialytic hypotension (IDH).
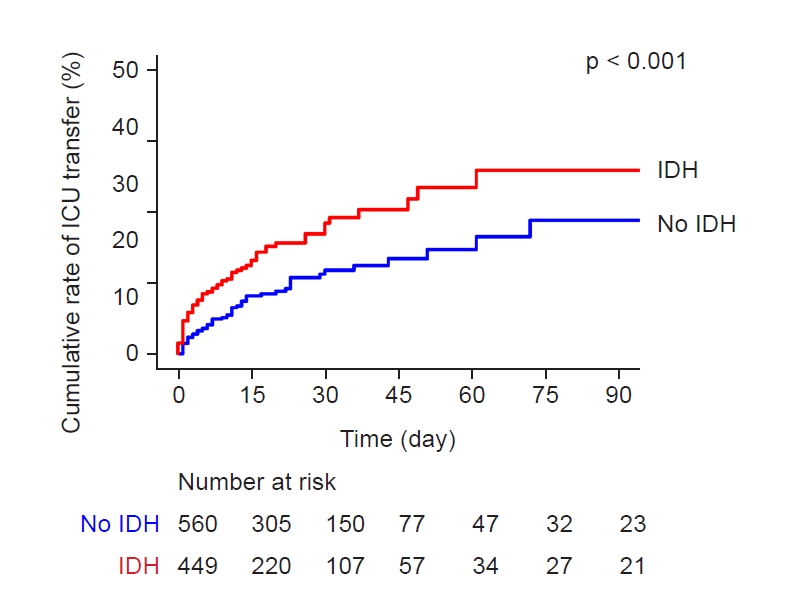
Table 1.
Table 2.
| Variable | Unadjusted HR (95% CI) | p-value | Adjusted HRa (95% CI) | p-value |
|---|---|---|---|---|
| Age (per 10 yr) | 1.00 (0.94–1.08) | 0.90 | 1.08 (1.00–1.17) | 0.049 |
| Male (vs. female) | 1.29 (1.03–1.63) | 0.03 | 1.30 (1.03–1.66) | 0.03 |
| Weight (per 1 kg) | 1.00 (1.00–1.01) | 0.33 | ||
| Initial SBP (per 10 mmHg) | 0.93 (0.89–0.97) | 0.001 | ||
| Initial DBP (per 10 mmHg) | 0.98 (0.98–1.05) | 0.51 | ||
| Pulse rate (per 10 beats/min) | 1.20 (1.14–1.27) | <0.001 | 1.17 (0.10–1.25) | <0.001 |
| Dialysis duration (per 1 hr) | 1.07 (0.73–1.57) | 0.70 | ||
| Blood flow rate (per 1 mL/hr) | 0.99 (0.98–0.99) | <0.001 | ||
| Ultrafiltration volume (per 1 L) | 0.92 (0.82–1.04) | 0.20 | ||
| Diagnosis of sepsis (vs. none) | 1.60 (1.28–2.01) | <0.001 | 1.26 (1.00–1.60) | 0.05 |
| Use of vasopressor (vs. none) | 1.39 (1.10–1.75) | 0.005 | ||
| Diabetes mellitus (vs. none) | 0.96 (0.73–1.26) | 0.77 | ||
| Hypertension (vs. none) | 1.17 (0.67–2.04) | 0.58 | ||
| Coronary artery disease (vs. none) | 0.93 (0.64–1.35) | 0.71 | 1.43 (0.97–2.12) | 0.07 |
| Atrial fibrillation (vs. none) | 0.84 (0.55–1.29) | 0.40 | ||
| Liver cirrhosis (vs. none) | 1.75 (1.34–2.29) | <0.001 | ||
| Chronic kidney disease (vs. none) | 0.67 (0.51–0.87) | 0.003 | ||
| Active malignancy (vs. none) | 2.99 (2.33–3.85) | <0.001 | 2.32 (1.78–3.02) | <0.001 |
| BUN (per 1 mg/dL) | 1.00 (1.00–1.01) | <0.001 | 1.00 (1.00–1.01) | 0.02 |
| Creatinine (per 1 mg/dL) | 0.92 (0.88–0.96) | <0.001 | 0.85 (0.80–0.91) | <0.001 |
| Sodium (per 1 mmol/L) | 1.00 (0.98–1.01) | 0.70 | 1.06 (1.04–1.09) | <0.001 |
| Potassium (per 1 mmol/L) | 0.94 (0.84–1.05) | 0.30 | ||
| Chloride (per 1 mmol/L) | 0.98 (0.96–0.99) | <0.001 | 0.92 (0.90–0.95) | <0.001 |
| Bicarbonate (per 1 mmol/L) | 0.97 (0.95–0.99) | <0.001 | 0.93 (0.91–0.95) | <0.001 |
| Total bilirubin (per 1 mg/dL) | 1.04 (1.03–1.05) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Albumin (per 1 g/dL) | 0.71 (0.61–0.84) | <0.001 | 0.78 (0.66–0.93) | 0.005 |
| CRP (per 1 mg/dL) | 1.02 (1.01–1.03) | <0.001 | ||
| IDH (vs. none) | 1.88 (1.50–2.36) | <0.001 | 1.30 (1.02–1.65) | 0.04 |
Table 3.
| Variable | Unadjusted HR (95% CI) | p-value | Adjusted HRa (95% CI) | p-value |
|---|---|---|---|---|
| Age (per 10 yr) | 0.96 (0.87–1.06) | 0.46 | ||
| Male (vs. female) | 1.10 (0.79–1.54) | 0.58 | ||
| Body weight (per 1 kg) | 1.02 (1.01–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| Initial SBP (per 10 mmHg) | 0.95 (0.89–1.01) | 0.10 | ||
| Initial DBP (per 10 mmHg) | 0.94 (0.84–1.04) | 0.22 | ||
| Pulse rate (per 10 beats/min) | 1.17 (1.08–1.27) | <0.001 | 1.14 (1.04–1.25) | 0.004 |
| Dialysis duration (per 1 hr) | 1.10 (0.59–2.04) | 0.77 | ||
| Blood flow rate (per 1 mL/hr) | 0.99 (0.99–1.00) | 0.06 | ||
| Ultrafiltration volume (per 1 L) | 1.09 (0.91–1.29) | 0.35 | ||
| Diagnosis of sepsis (vs. none) | 1.22 (0.88–1.69) | 0.23 | ||
| Use of vasopressor (vs. none) | 1.42 (1.01–2.00) | 0.045 | ||
| Diabetes mellitus (vs. none) | 0.89 (0.60–1.33) | 0.57 | ||
| Hypertension (vs. none) | 0.56 (0.18–1.75) | 0.32 | ||
| Coronary artery disease (vs. none) | 1.16 (0.74–1.88) | 0.55 | 1.60 (0.97–2.65) | 0.07 |
| Atrial fibrillation (vs. none) | 0.74 (0.38–1.46) | 0.39 | ||
| Liver cirrhosis (vs. none) | 1.44 (0.94–2.20) | 0.10 | ||
| Chronic kidney disease (vs. none) | 0.84 (0.59–1.22) | 0.36 | ||
| Active malignancy (vs. none) | 1.23 (0.88–1.71) | 0.23 | ||
| BUN (per 1 mg/dL) | 1.00 (1.00–1.01) | 0.047 | 1.00 (1.00–1.01) | 0.10 |
| Creatinine (per 1 mg/dL) | 0.91 (0.85–0.98) | 0.01 | 0.86 (0.79–0.94) | <0.001 |
| Sodium (per 1 mmol/L) | 0.99 (0.98–1.02) | 0.99 | 1.06 (1.03–1.10) | <0.001 |
| Potassium (per 1 mmol/L) | 0.88 (0.75–1.04) | 0.13 | ||
| Chloride (per 1 mmol/L) | 0.98 (0.96–1.00) | 0.03 | 0.94 (0.91–0.97) | <0.001 |
| Bicarbonate (per 1 mmol/L) | 0.98 (0.95–1.01) | 0.22 | 0.94 (0.91–0.97) | <0.001 |
| Total bilirubin (per 1 mg/dL) | 1.03 (1.02–1.05) | <0.001 | 1.03 (1.01–1.05) | <0.001 |
| Albumin (per 1 g/dL) | 0.85 (0.66–1.08) | 0.17 | ||
| CRP (per 1 mg/dL) | 1.01 (1.00–1.03) | 0.16 | ||
| IDH, (vs. none) | 1.70 (1.22–2.36) | 0.002 | 1.43 (1.02–2.02) | 0.04 |
Table 4.
| Variable | Unadjusted OR (95% CI) | p-value | Adjusted ORa (95% CI) | p-value |
|---|---|---|---|---|
| Age (per 10 yr) | 1.23 (1.12–1.35) | <0.001 | 1.26 (1.15–1.37) | <0.001 |
| Male (vs. female) | 0.83 (0.62–1.12) | 0.23 | 0.81 (0.62–1.06) | 0.13 |
| Body weight (per 1 kg) | 0.99 (0.98–1.00) | 0.17 | ||
| Initial SBP (per 10 mmHg) | 1.11 (1.05–1.17) | <0.001 | 1.10 (1.04–1.16) | <0.001 |
| Pulse rate (per 10 beats/min) | 1.25 (1.16–1.35) | <0.001 | 1.26 (1.17–1.35) | <0.001 |
| UF volume (per 1 L) | 1.09 (0.93–1.29) | 0.27 | ||
| UF volume per weight (mL/hr/kg) | 1.01 (1.00–1.02) | 0.09 | ||
| Diagnosis of sepsis (vs. none) | 1.27 (0.92–1.76) | 0.15 | ||
| Use of vasopressor (vs. none) | 1.15 (0.87–1.52) | 0.32 | ||
| Diabetes mellitus (vs. none) | 1.24 (0.88–1.75) | 0.22 | ||
| Hypertension (vs. none) | 1.64 (0.80–3.35) | 0.17 | ||
| Coronary artery disease (vs. none) | 1.04 (0.67–1.63) | 0.85 | ||
| Liver cirrhosis (vs. none) | 1.62 (1.07–2.47) | 0.02 | 1.81 (1.24–2.65) | 0.002 |
| Active malignancy (vs. none) | 1.39 (1.05–1.84) | 0.02 | 1.34 (1.02–1.77) | 0.03 |
| Total bilirubin (per 1 mg/dL) | 1.01 (1.00–1.03) | 0.14 | ||
| Albumin (per 1 g/dL) | 0.61 (0.49–0.76) | <0.001 | 0.60 (0.49–0.74) | <0.001 |
| C-reactive protein (per 1 mg/dL) | 0.99 (0.98–1.01) | 0.57 |
References
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 388 View
- 19 Download
- ORCID iDs
-
Yeong-Won Park

https://orcid.org/0009-0003-4422-8226Donghwan Yun

https://orcid.org/0000-0001-6566-5183Yeojin Yu

https://orcid.org/0009-0004-2988-0440Sang Hyun Kim

https://orcid.org/0009-0003-4495-6792Sehoon Park

https://orcid.org/0000-0002-4221-2453Yong Chul Kim

https://orcid.org/0000-0003-3215-8681Dong Ki Kim

https://orcid.org/0000-0002-5195-7852Kook-Hwan Oh

https://orcid.org/0000-0001-9525-2179Kwon Wook Joo

https://orcid.org/0000-0001-9941-7858Yon Su Kim

https://orcid.org/0000-0003-3091-2388Seong Geun Kim

https://orcid.org/0000-0002-8831-9838Seung Seok Han

https://orcid.org/0000-0003-0137-5261 - Related articles
-
COVID-19 incidence and outcomes among patients with kidney replacement therapy2023 September;42(5)
Nephrology consultation improves the clinical outcomes of patients with acute kidney injury



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















