| Kidney Res Clin Pract > Epub ahead of print |
Abstract
Background
Methods
Results
Supplementary Materials
Notes
Funding
This study was supported by the Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, the Young Investigator Research Grant from the Korean Society of Nephrology (K-NRF-Chongkundang, 2020), and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1F1A1073317).
Data sharing statement
The data presented in this study are available upon reasonable request from the corresponding author.
Authors’ contributions
Conceptualization: SRK, IYK, SBL, DWL
Funding acquisition: SRK
Investigation, Methodology: SRK, YSK, MJK
Data curation, Formal analysis: YSK, JMH, SJK, BMY, MJK, SRK, BHC, DY
Supervision: IYK, SBL, DWL
Writing–original draft: SRK
Writing–review & editing: SRK, BHC, DY, IYK, SBL, DWL
All authors read and approved the final version of the manuscript.
Figure 1.
Characteristics of diet-induced obesity (DIO) mice with ischemia-reperfusion (IR) injury and serum and urine measurements.
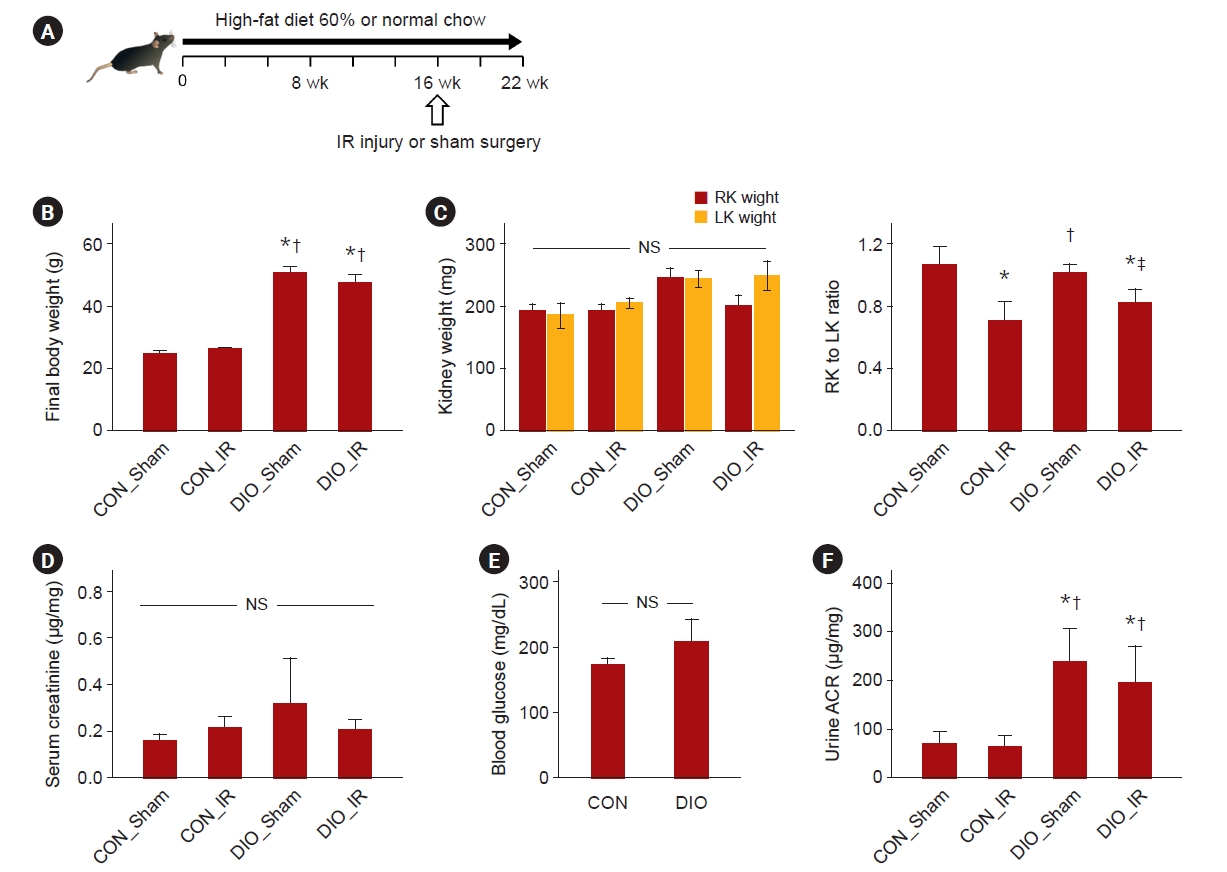
Figure 2.
Senescence in the ischemia-reperfusion (IR) injured kidneys of diet-induced obese (DIO) mice.
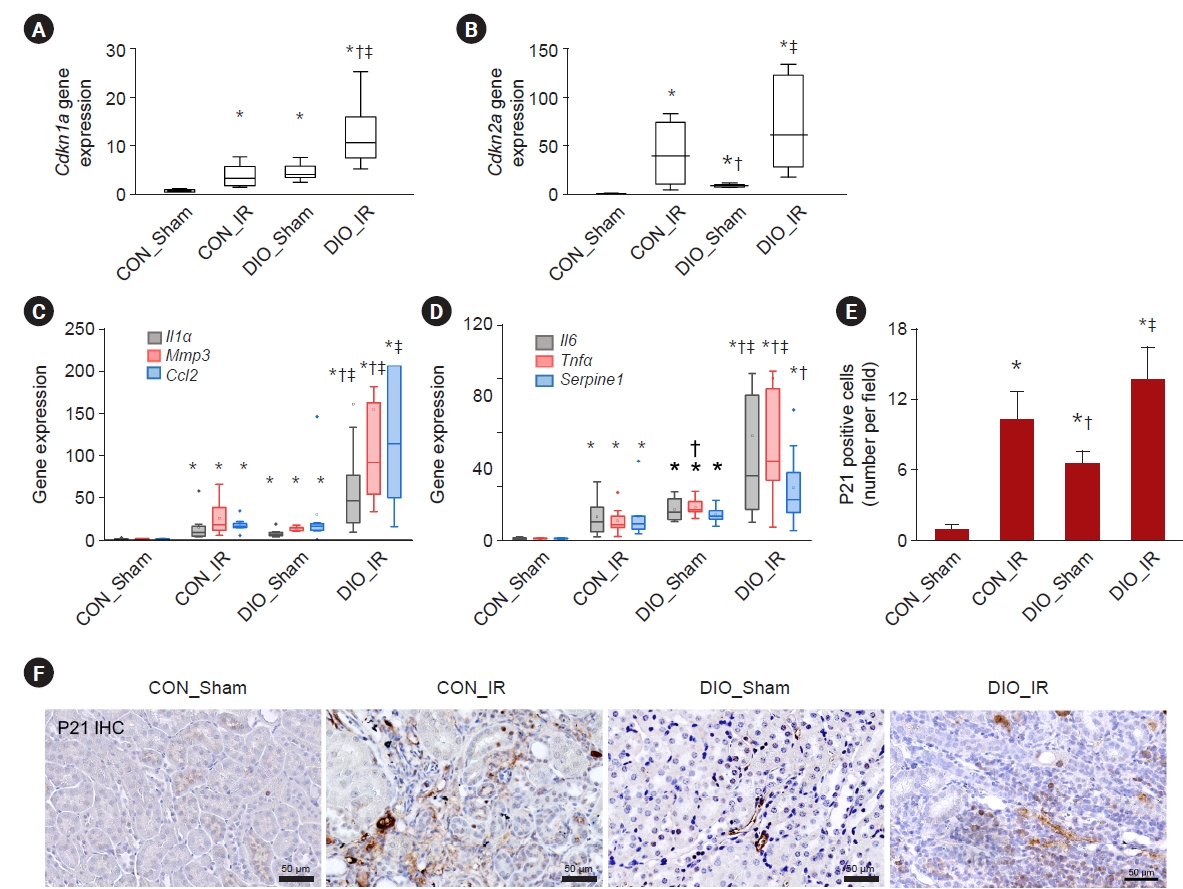
Figure 3.
Renal tubular injury, fibrosis, and inflammation in the ischemia-reperfusion (IR) injured kidneys of diet-induced obese (DIO) mice.
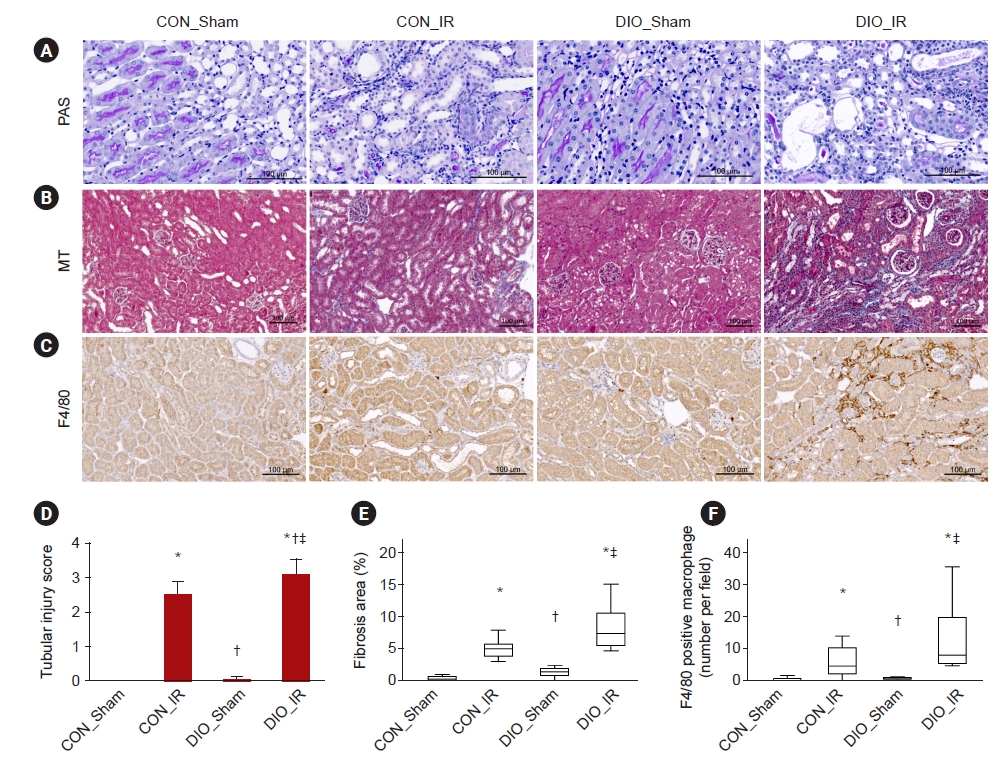
Figure 4.
Senescence expression in the epididymal (E) and perirenal (K) fat depots of mice undergoing sham or ischemia-reperfusion (IR) injury with normal chow or a high-fat diet (diet-induced obesity, DIO).
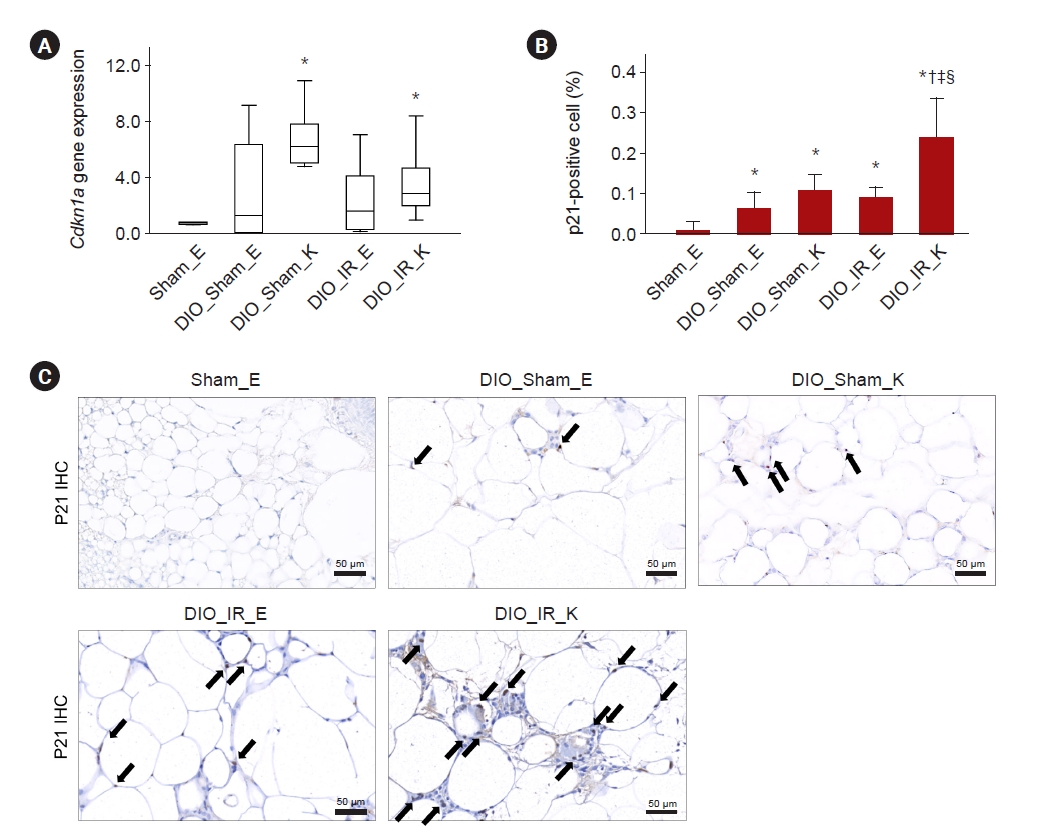
Figure 5.
Expression of adiponectin and cytokines in the epididymal (E) and perirenal (K) fat depots of mice undergoing sham or ischemia-reperfusion (IR) injury with normal chow or a high-fat diet (diet-induced obesity, DIO).

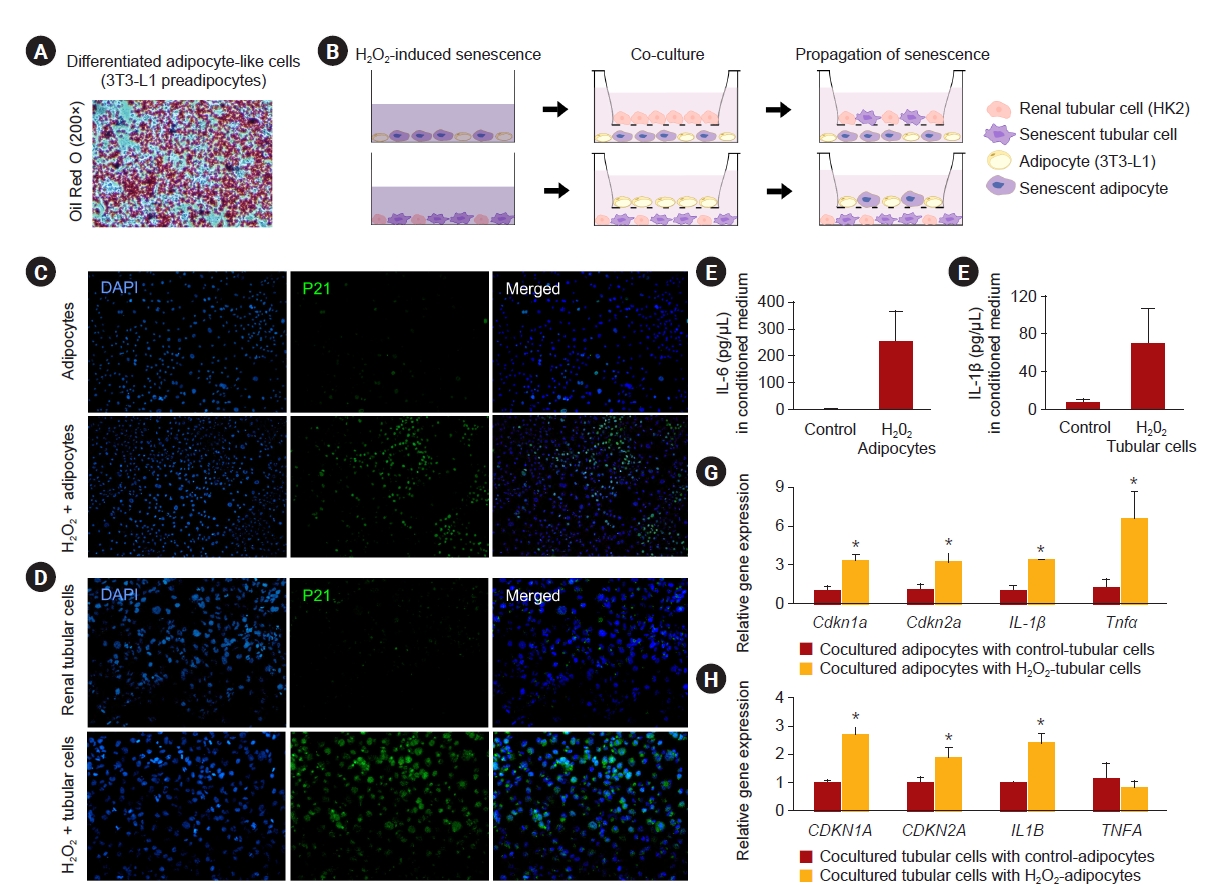
Figure 6.
In vitro study of the coculture system using adipocytes (3T3-L1s) and renal tubular cells (HK2s).
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 305 View
- 3 Download
- ORCID iDs
-
Seo Rin Kim

https://orcid.org/0000-0003-3552-0519Young-Suk Kim

https://orcid.org/0000-0002-7332-8774Je Min Hyeon

https://orcid.org/0000-0003-2921-8292Su Ji Kim

https://orcid.org/0000-0002-5494-5451Byung Min Ye

https://orcid.org/0000-0002-0751-8895Min Jeong Kim

https://orcid.org/0000-0003-2454-9201Byung Hyun Choi

https://orcid.org/0000-0002-6269-5595Dongwon Yi

https://orcid.org/0000-0003-3574-036XIl Young Kim

https://orcid.org/0000-0002-1731-6357Soo Bong Lee

https://orcid.org/0000-0002-3388-7993Dong Won Lee

https://orcid.org/0000-0003-0282-484X - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















