| Kidney Res Clin Pract > Volume 43(1); 2024 > Article |
|
Abstract
Supplementary Materials
Acknowledgments
Notes
Funding
This research was supported by a cooperative research fund from the Korean Society of Nephrology (2022).
Data sharing statement
The data presented in this study are available upon reasonable request from the corresponding author.
AuthorsŌĆÖ contributions
Conceptualization: YKK, HEY
Data curation, Formal analysis: SAJ
Funding acquisition, Methodology: HEY
Investigation: KMK, THB, YAH, SDH, SRC, HL, JHK, SHK, THK, HSK, CYY, KK, SHA, HEY
WritingŌĆōoriginal draft: KMK, HEY
WritingŌĆōreview & editing: THB, YAH, SDH, SRC, HL, JHK, SHK, THK, HSK, CYY, KK, SHA, YKK
All authors read and approved the final manuscript.
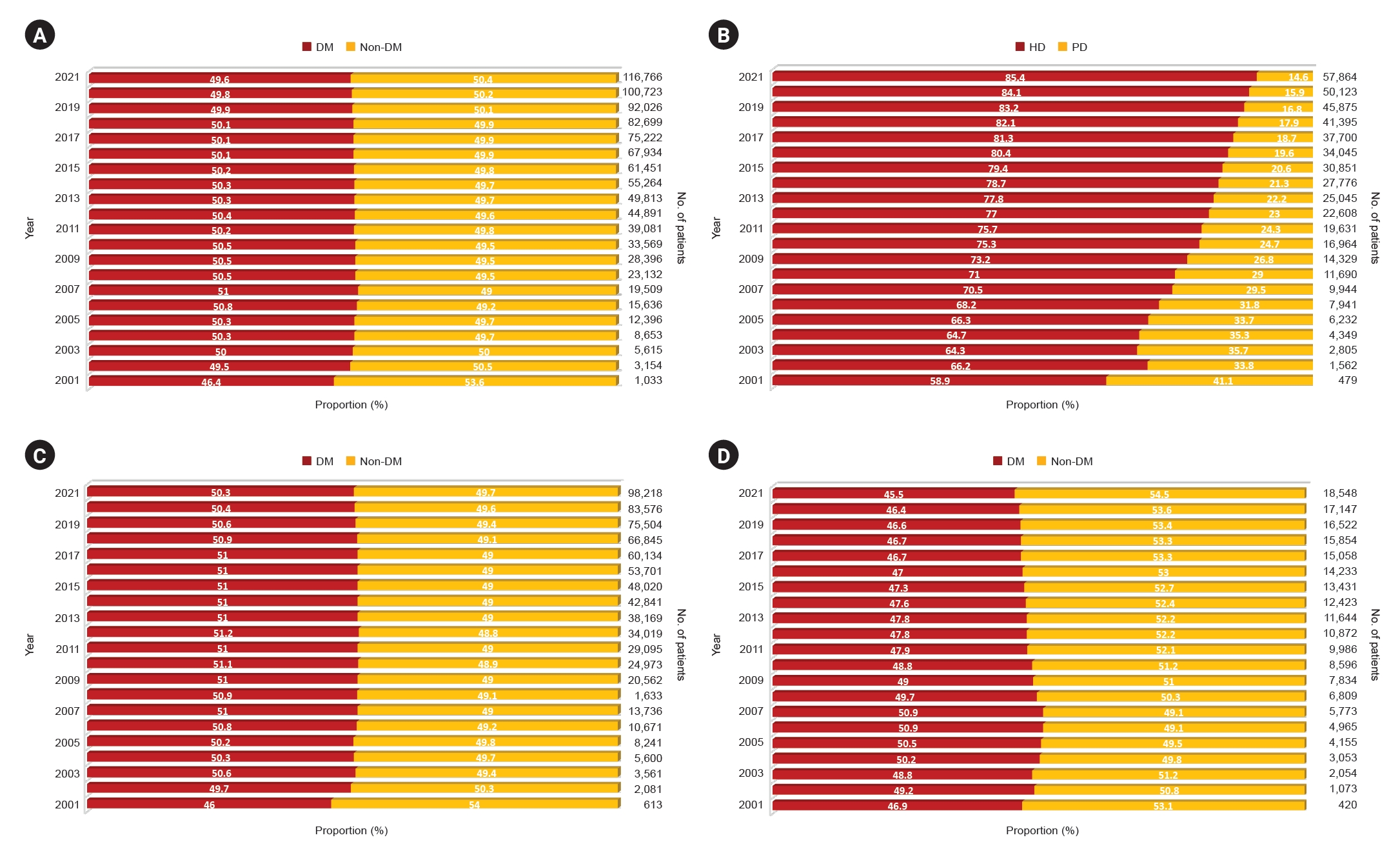
Figure┬Ā1.
Changes in the proportion of patients with diabetic CKD 5D.
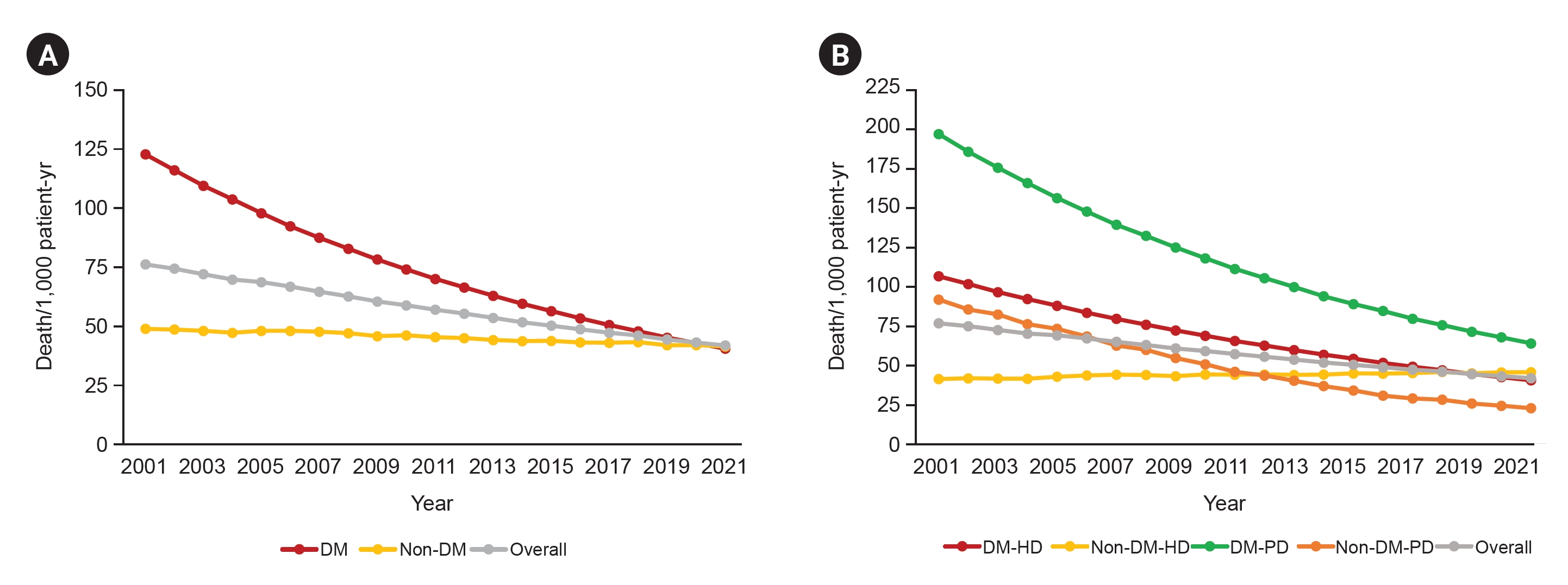
Figure┬Ā2.
All-cause mortality in patients with diabetic CKD 5D.
Figure┬Ā3.
Age and sex distribution of diabetic CKD 5D in 2021.

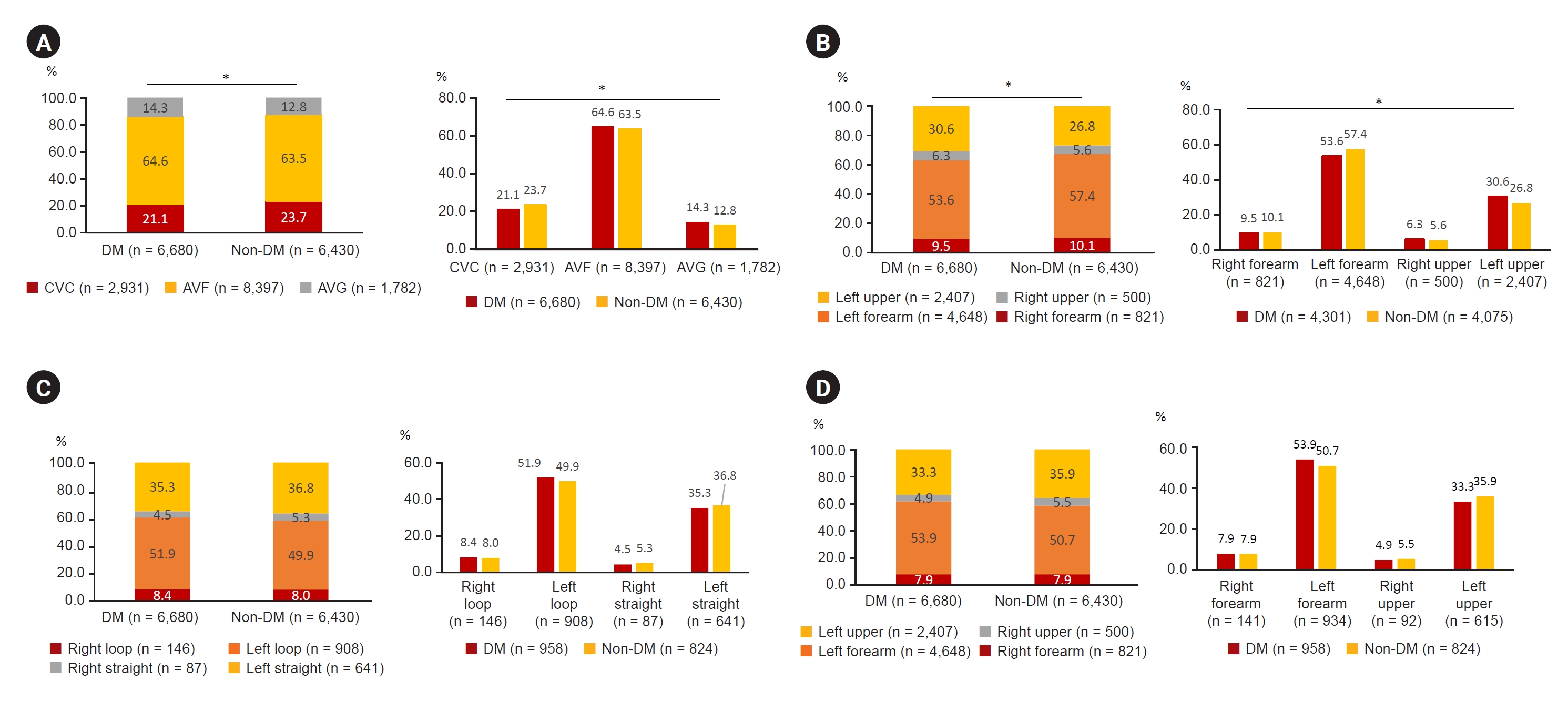
Figure┬Ā4.
Characteristics of HD access of diabetic CKD 5HD patients.
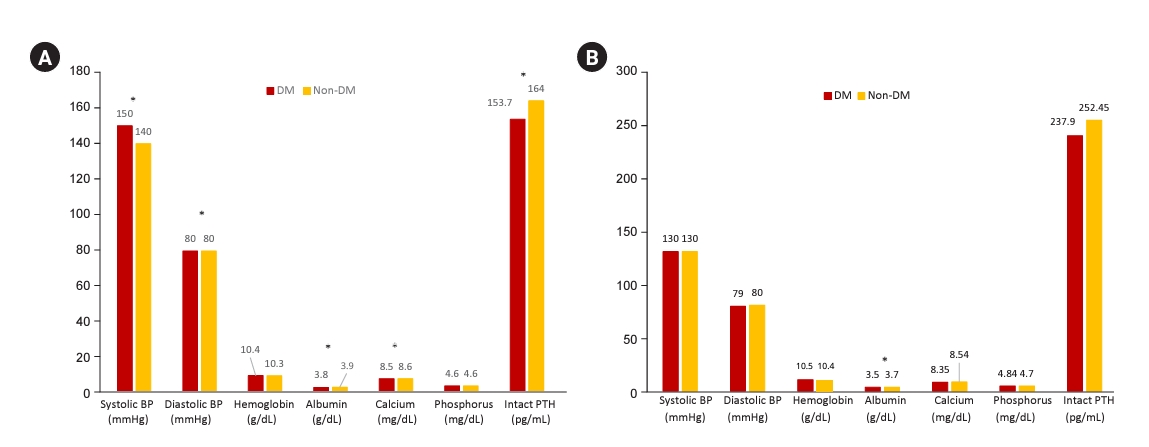
Figure┬Ā5.
BP and laboratory values in diabetic CKD 5HD and CKD 5PD patients.
Figure┬Ā6.
Characteristics of diabetic CKD 5PD patients.

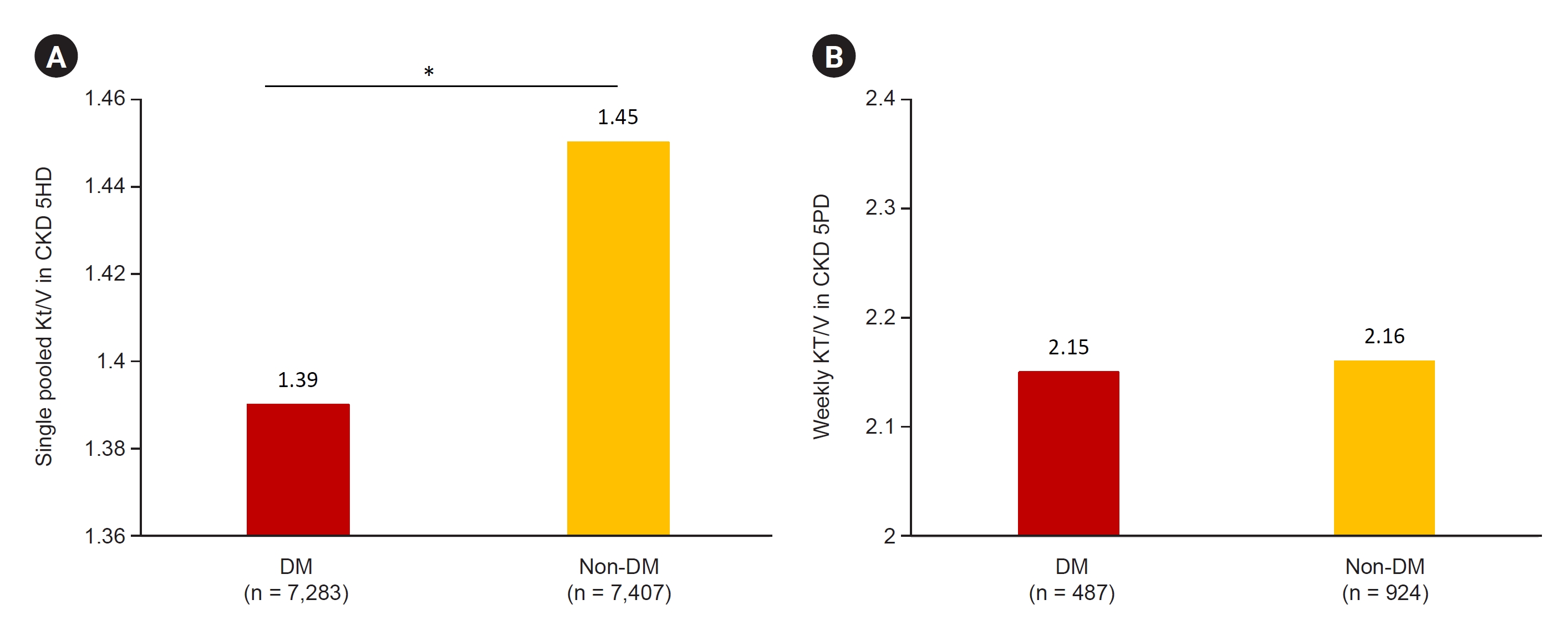
Figure┬Ā7.
Dialysis adequacy in diabetic CKD 5HD and CKD 5PD patients.
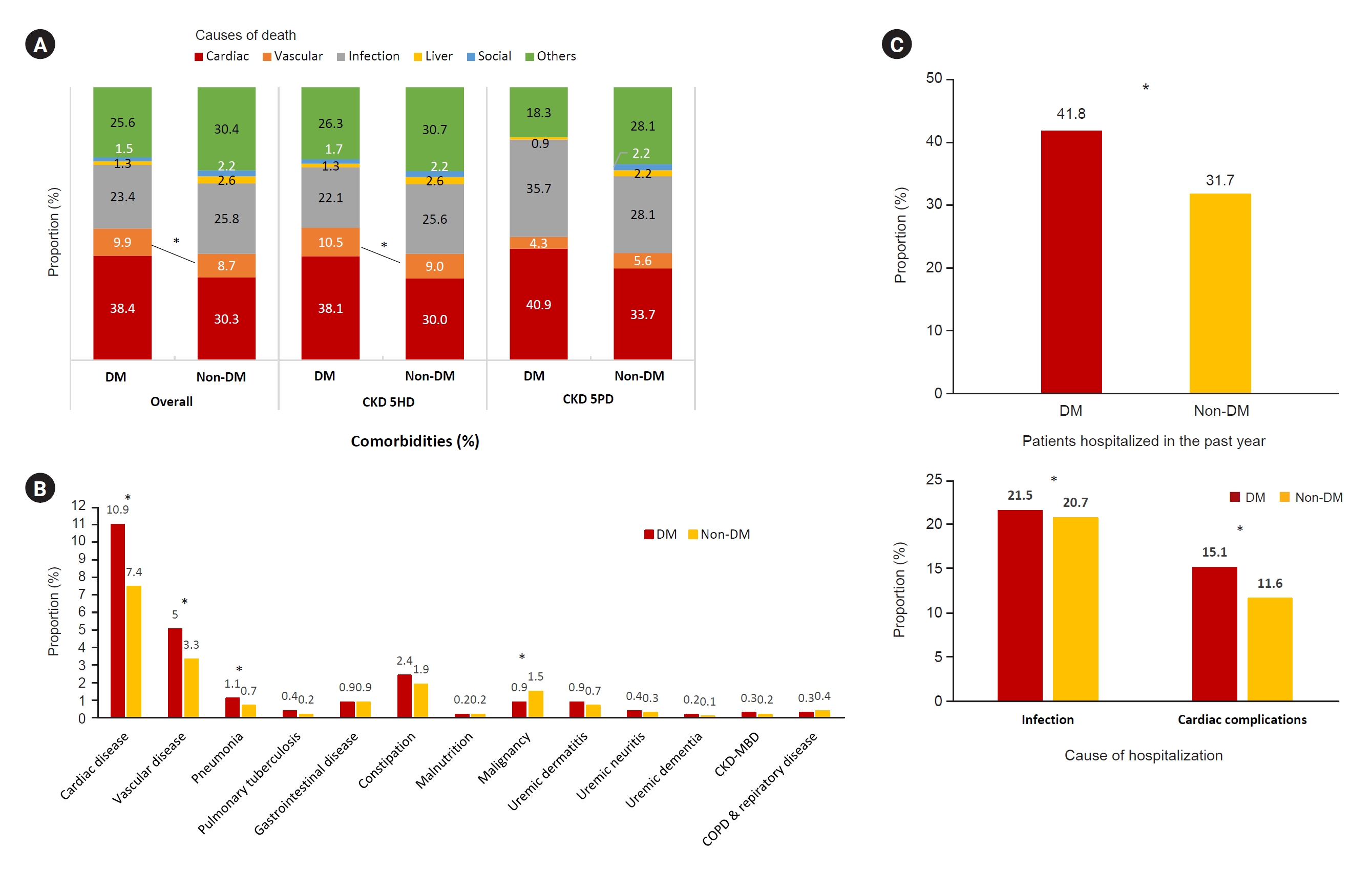
Figure┬Ā8.
Causes of death, comorbidities, and hospitalization in patients with diabetic CKD 5D.
References
- TOOLS
-
METRICS

-
- 2 Crossref
- 0 Scopus
- 1,078 View
- 105 Download
- ORCID iDs
-
Kyeong Min Kim

https://orcid.org/0000-0002-5414-4339Seon A Jeong

https://orcid.org/0009-0009-1615-5983Tae Hyun Ban

https://orcid.org/0000-0002-2884-4948Yu Ah Hong

https://orcid.org/0000-0001-7856-4955Seun Deuk Hwang

https://orcid.org/0000-0003-0074-6972Sun Ryoung Choi

https://orcid.org/0000-0002-9668-3349Hajeong Lee

https://orcid.org/0000-0002-1873-1587Ji Hyun Kim

https://orcid.org/0000-0001-8477-0157Su Hyun Kim

https://orcid.org/0000-0003-3382-528XTae Hee Kim

https://orcid.org/0000-0002-3001-234XHo-Seok Koo

https://orcid.org/0000-0001-7856-8083Chang-Yun Yoon

https://orcid.org/0000-0001-8545-9344Kiwon Kim

https://orcid.org/0000-0002-2885-0053Seon Ho Ahn

https://orcid.org/0009-0002-2603-9184Yong Kyun Kim

https://orcid.org/0000-0002-1871-3549Hye Eun Yoon

https://orcid.org/0000-0002-6347-7282 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print















