Introduction
Kidney transplantation (KT) is the main treatment option for patients with end-stage kidney disease (ESKD). However, it is limited by the low number of donor organs [
1]. Moreover, donor–recipient size mismatching is a common problem in KT given the various types of donors [
2]. Donor–recipient size mismatching is divided into two main types. The first type is when a large recipient receives a small donor’s kidney (e.g., when the recipient is an adult and the deceased donor is a child). In this case, the risk of graft loss is high because of hyperfiltration injury [
3–
5]. The second type is when a small child receives a kidney from an adult. In this case, various medical problems can occur, including hemodynamic imbalance, low perfusion into the transplant kidney, and increased heart burden on the recipient [
2] and poor long-term prognosis of transplant kidneys [
6]. Age- and size-matched pediatric donors are difficult to find in pediatric KT. Therefore, kidneys from adult donors are used in most pediatric recipients, resulting in size mismatching. Accordingly, this study aimed to determine the effect of donor–recipient size mismatch on the long-term survival rate of transplant kidneys in pediatric KT.
Methods
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of Kyungpook National University Hospital (No. 2021-10-005). Informed consent was waived because of the study’s retrospective design.
Study population and data collection
A total of 241 patients who received KT at three national university hospitals from 2000 to 2019 were enrolled. All patients were less than 19 years of age, followed up for more than 1 year, and had available medical data of donors. The medical records and Korean Network for Organ Sharing data of all patients were retrospectively reviewed. Information of recipients and donors including age; sex; body weight; height; body surface area (BSA); graft weight; causes of ESKD; duration and modality of dialysis before KT; cold/warm ischemic time; human leukocyte antigen (HLA) matching; maintenance immunosuppressive regimen; occurrence of delayed graft function (DGF); estimated glomerular filtration rate (eGFR) at the time of discharge during KT, 1 year after KT, and last follow-up; and renal events after KT (restart of dialysis by graft failure) was collected. The surgical method used at the time of transplantation (extraperitoneal vs. transabdominal approach) was also investigated. The Mosteller formula, square root [(height (cm) × weight (kg))/3,600], was used to calculate the BSA. Meanwhile, the revised Schwartz formula, 0.413 × height (cm)/serum creatinine (mg/dL), was used to calculate the eGFR in children receiving KT. The donor–recipient body weight ratio (DRBWR) was calculated by dividing the donor’s body weight by the recipient’s body weight at the time of KT. The donor–recipient body surface area ratio (DRBSR) was calculated by dividing the donor’s BSA by the recipient’s BSA at the time of KT.
Statistical analysis
In descriptive analysis, categorical variables were presented as frequencies and percentages, and continuous variables were presented as mean ± standard deviation or median (interquartile range). The statistical difference was evaluated using the chi-square test or Fisher exact test for categorical variables, and analysis of variance or Kruskal-Wallis test for continuous variables. The Pearson correlation coefficient and linear regression model were used for statistical analyses between two continuous variables. As survival analysis, Fine and Gray’s subdistribution hazard model was applied for estimating cumulative incidence for graft failure, considering competing risk including death not related to KT. Statistical significance was considered at p-value of <0.05. R version 4.1.1 (R Foundation for Statistical Computing) was used to conduct all statistical analysis.
Results
A total of 241 pediatric patients who received KT were enrolled. At the time of transplantation, the mean age of patients was 11.65 ± 4.63 years, and the ratio of males and females was 149:92. The age distribution was as follows: 25 patients were aged under 5 years, 60 patients were aged 5–10 years, 86 patients were aged 10–15 years, and 70 patients were aged over 15 years. Congenital anomalies of the kidney and urinary tract were the most common cause of ESKD, accounting for 42.1% of the total. The mean body weight, height, and BSA of patients at the time of transplantation were 34.31 ± 16.85 kg, 134.99 ± 25.77 cm, and 1.12 ± 0.37 m
2, respectively. Two patients had a history of previous KT, and 58 patients (24.1% of the total) received preemptive KT. The remaining patients underwent dialysis for an average of 21.56 ± 20.47 months before KT. Peritoneal dialysis was the most common modality of dialysis. KT was performed via the transabdominal approach in 76.3% of the total, and triple regimen including tacrolimus, mycophenolate mofetil, and prednisolone was used as the maintenance immunosuppressive regimen in 79.7% of the total. The mean cold and warm ischemic times at the time of KT were 112.55 ± 106.67 minutes and 39.35 ± 14.23 minutes, respectively, and four patients (1.7%) developed DGF. The mean follow-up duration was 96.49 ± 52.98 months (
Table 1).
The mean age of donors was 34.74 ± 14.91 years, and the ratio of males and females was 105:136. Approximately 81.7%, 1.2%, and 17.0% of all transplant donors were identified as living-related, living-unrelated, and deceased donors, respectively. Among the living-related donors, the recipient’s mother and father accounted for 53.3% and 34.5% of the total, respectively. The mean body weight, height, and BSA of donors were 56.53 ± 16.73 kg, 156.22 ± 21.02 cm, and 1.56 ± 0.34 m
2, respectively. The mean eGFR of donors and mean weight of transplanted kidneys were 86.13 ± 41.40 mL/min/1.73 m
2 and 153 g, respectively (
Table 2).
In the
Table 3, we compared the baseline characteristics of recipients and donors according to DRBWR category (<1, ≥1 to <3, and ≥3). In the group with DRBWR of ≥3, the recipients were significantly younger and had lower body weight than that in the other groups. In addition, transabdominal approaches were widely used in the KT, but the cold ischemic time was significantly shorter than that in the other groups. On the other hand, the donors in the group with DRBWR of ≥3 had the higher rate of living-related donor and higher body weight than that in the other groups.
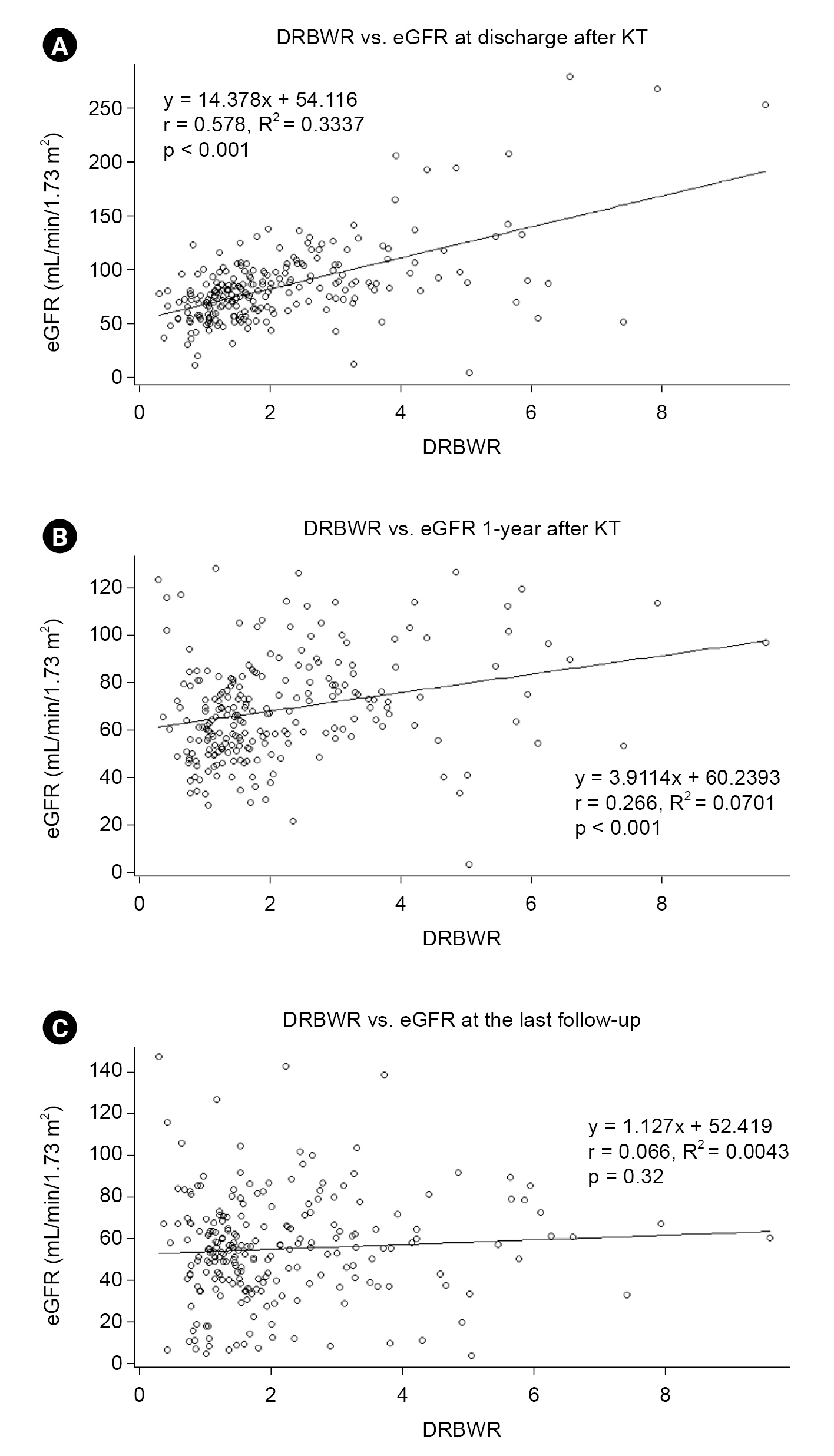
The association between DRBWR and eGFR of transplant kidneys was analyzed at the time of discharge immediately after KT (
Fig. 1A), 1 year after KT (
Fig. 1B), and last follow-up (96.49 ± 52.98 months after KT) (
Fig. 1C). The DRBWR was significantly positively associated with the eGFR of transplant kidneys at the time of discharge after KT (Pearson’s r = 0.578, p < 0.001) and 1 year after KT (r = 0.266, p < 0.001). This finding shows that the larger the body weight of the donor compared with the recipient, the greater the eGFR of the transplant kidney. However, this association weakened with extension of the follow-up period and could not be confirmed at the last follow-up (r = 0.066, p = 0.32). Similarly, these results were also confirmed in the association analysis between DRBSR and eGFR of transplant kidneys (
Fig. 2). The DRBSR was significantly positively associated with the eGFR at the time of discharge after KT (Pearson’s r = 0.584, p < 0.001) (
Fig. 2A) and 1 year after KT (r = 0.269, p < 0.001) (
Fig. 2B). However, no significant association was observed at the last follow-up (r = 0.069, p = 0.30) (
Fig. 2C).
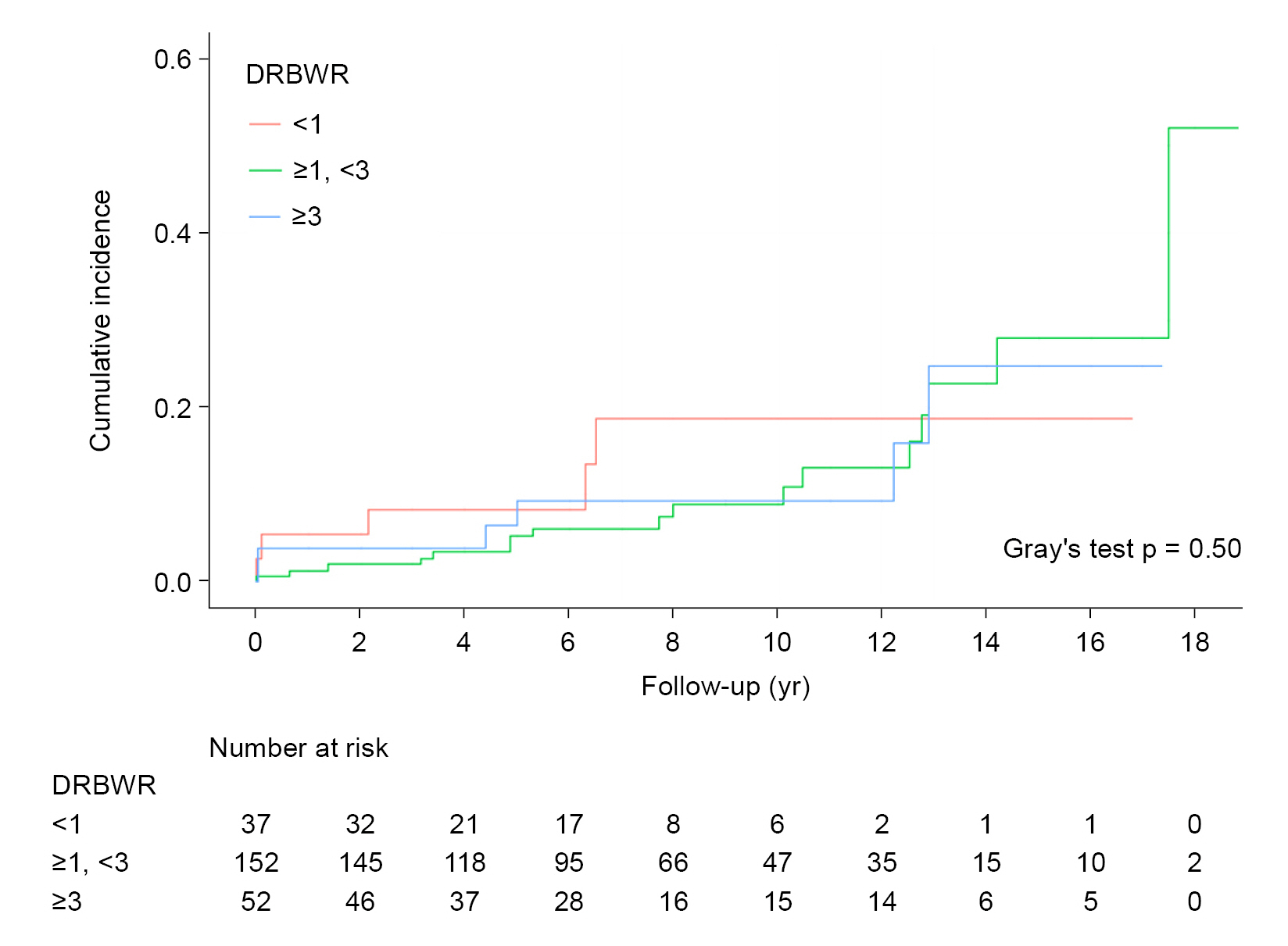
The survival curves of Fine and Gray’s subdistribution hazard model were expressed as the cumulative incidence according to DRBWR category (<1, ≥1 to <3, and ≥3) (
Fig. 3). No significant differences were observed between each curve (Gray test p = 0.50 in
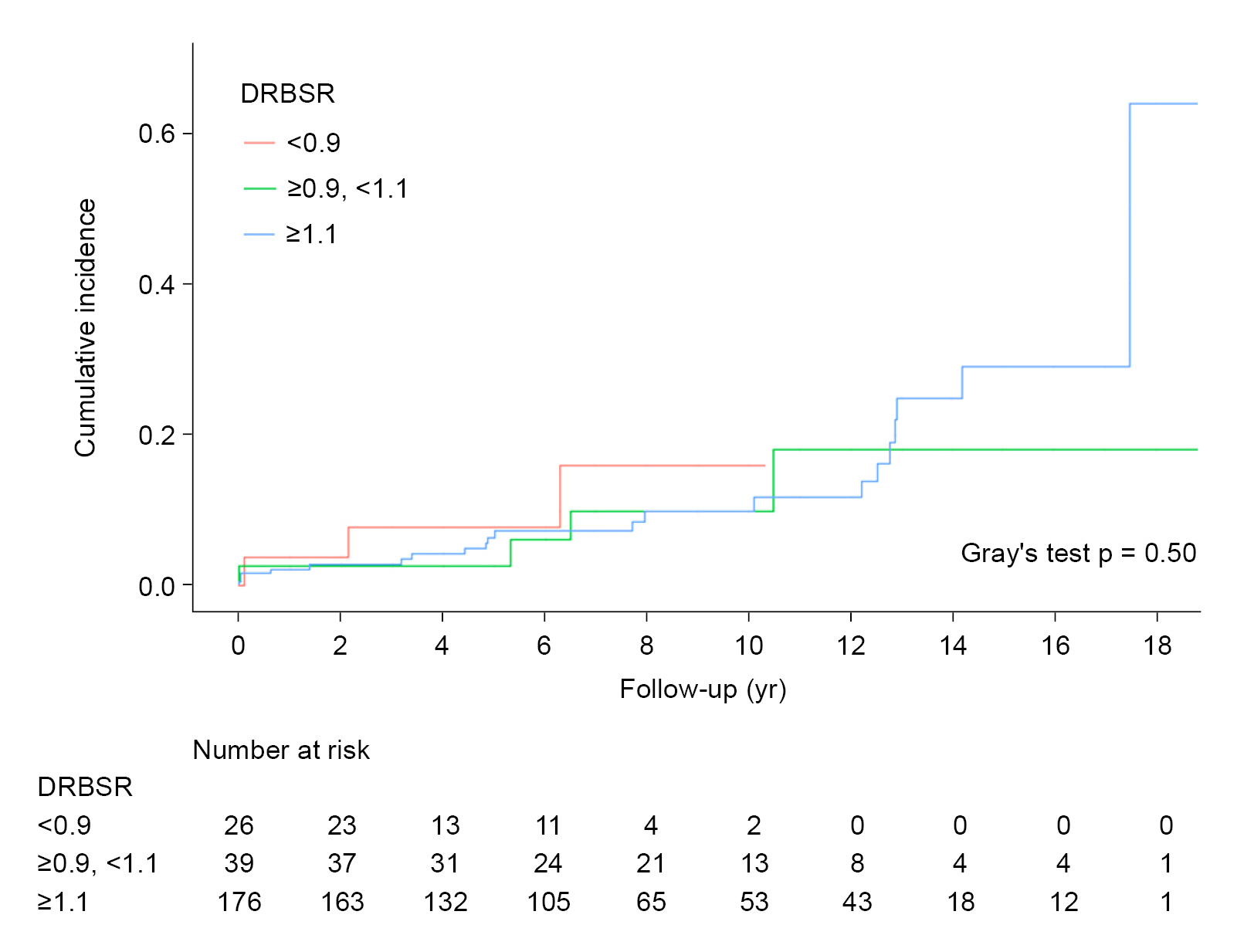
Fig. 3). The curves did not differ significantly from each other (Gray test p = 0.50) compared with the DRBWR results in the cumulative incidence curves plotted according to DRBSR category (<0.9, ≥0.9 to <1.1, and ≥1.1) (
Fig. 4).
Discussion
This study showed that donor–recipient size mismatching in pediatric KT can affect the transplant kidney’s function until 1 year immediately after transplantation, but not its long-term survival rate.
The number of KT in Korea is increasing every year and an average of 48.5 cases of pediatric KT have been performed annually for the last 10 years [
7]. A number of considerations need to be examined when choosing the most ideal donor for pediatric KT. For example, extended criteria donors (e.g., donation after cardiac death), donors with acute kidney injury, and HLA- or ABO-mismatched donors are usually not recommended in pediatric KT [
1,
8]. Moreover, pediatric recipients inevitably receive kidneys from adults because deceased pediatric donors with similar size and age as the recipients are extremely difficult to find [
2]. In this study, 200 living donors (82.9% of total donors) were all adults, showing that donor–recipient size mismatch commonly occurs in pediatric KT.
During childhood, the length and volume of the kidney grow to the adult size according to age and are known to be related to the height and weight [
9,
10]. Based on a prospective observational study of 437 normal Korean children aged between 0 and <13 years, Oh et al. [
9] reported that there were good correlations between kidney length and various somatic values, including body weight, height, and BSA and suggested the following equation for the reference values of kidney length for Korean children: kidney length of the right kidney (cm) = 0.051 × height (cm) + 2.102; kidney length of the left kidney (cm) = 0.051 × height (cm) + 2.280. In addition, Kim et al. [
10] also reported that renal length and volume in Korean children showed the strongest significant correlation with their height and weight, respectively. Therefore, donor–recipient size mismatching in KT implies donor–recipient kidney size mismatching.
Various medical problems occur when a kidney from a small pediatric donor is provided to an adult recipient [
3–
5,
11]. The same situation can be observed in the case of adolescent recipients receiving kidneys from deceased pediatric donors. In this case, glomerular hypertrophy occurs probably because of hyperfiltration damage caused by nephron underdosing, leading to a low long-term survival rate of the transplant kidney [
4,
5]. The risk of graft loss significantly increases when the DRBSR is less than 0.9 or the donor’s weight is 30 kg less than the recipient’s weight [
12,
13].
The effects of donor–recipient size mismatch on graft outcome in pediatric KT (a small pediatric recipient and a large adult donor) have been reported in previous studies [
6,
14–
16]. According to the North American Pediatric Renal Trials and Collaborative Studies, acute tubular necrosis (ATN) occurred in 10% of infants who received adult kidneys, requiring dialysis posttransplantation, and the infants showed poor long-term survival rates [
6]. In addition, ATN occurred more frequently in small children or infants who received adult kidneys than adults who received KT [
6]. The occurrence of ATN can theoretically be expected considering the marked hemodynamic difference between the cardiovascular system of pediatric recipients and the kidneys from adults. An adult’s cardiac output at rest is approximately 5 L/min, and both kidneys receive 20% of the cardiac output. Therefore, one kidney is supplied with 500 mL of blood per minute. On the other hand, the total blood volume of a child weighing 10 kg is about 800 mL (80 mL/kg), so one transplant kidney from an adult requires approximately 62% of the total blood volume of a pediatric recipient. Therefore, the transplant kidney from adult donors shows hypoperfusion status immediately after pediatric KT, and ATN often occurs. In 2000, according to their analysis of a single center and United Network for Organ Sharing database, Sarwal et al. [
14] emphasized the importance of preventing ATN after KT. They reported that adult-size kidneys without ATN from living and deceased donors lead to remarkably better long-term graft outcomes for small children; moreover, they created protocols for ATN prevention including long-term aggressive fluid management (3,000 ± 500 mL/m
2/day with mean sodium content of 10 ± 4 mEq/kg/day) for a mean of 9 months after KT and maintenance of higher blood pressure (up to the 95th percentile for age and sex) until 6 months after KT [
15]. However, based on the analysis of data from 99 pediatric patients who received KT under the age of 10 years, Pape et al. [
16,
17] reported that the long-term survival rate of transplant kidneys was significantly lower than that in the case of donors under the age of 16 years when a kidney was transplanted from a donor aged 16 years or older; they concluded that age-matched exchange is essential for pediatric KT. Therefore, the controversy over the ideal donor in pediatric KT continued until the 2000s.
In 2010, Goldsmith et al. [
18] analyzed the data from 23 low-weight pediatric patients who received KT and reported no difference in the short-term survival rate of transplant kidneys between low-weight and high-weight donors. In addition, Lee et al. [
19] reported the successful graft survival rate based on single-center data in the United States, in spite of donor–recipient size mismatch in pediatric KT. They reported that the graft survival rate of adult-sized transplant kidney in pediatric recipients under 20 kg was 98.4%, 96.6%, and 84.2% at 1, 5, and 10 years, respectively; moreover, they emphasized the importance of careful perioperative management including adequate fluid resuscitation and use of pressors. Based on an analysis of single-center data in Europe, Amesty et al. [
20] also reported that adult-sized kidneys can be successfully transplanted to children under 20 kg of weight with no differences in graft survival, GFR, proteinuria, and rejection episodes.
Similar to previous reports in the United States and Europe, the present study confirmed that donor–recipient size mismatch is no longer a factor that adversely affects the long-term graft survival rate of pediatric KT in Korea. In addition, the larger the donor’s weight or BSA, the higher the GFR immediately after KT and until the first year after KT. However, this correlation disappeared during the final follow-up. These results showed that kidneys from adults with large bodies temporarily showed a relatively higher GFR compared with the recipient’s size; however, they adapted to the recipient’s size over time. Survival analysis using Fine and Gray’s subdistribution hazard model showed no difference in long-term graft survival between groups with more than three times the body weight of donors, and groups with more than one times and less than three times the body weight of donors, and groups with less than one times the body weight of donors compared with recipients. These results were the same in comparison based on BSA. Accordingly, Lepeytre et al. [
21] reported that even if size mismatch occurs, the donor shows excellent long-term survival rate if the donor is young; thus, donor age is a much stronger determinant of graft survival rate than size mismatch.
Unfortunately, although donor–recipient size mismatch was identified as a factor that adversely affected the long-term survival rate of transplant kidneys in previous reports published before 2010, the present study could not confirm the specific reasons why it did not show similar results in recent reports. In general, methods were used to minimize the ultrafiltration volume of dialysis before KT and to keep the central venous pressure and blood pressure high at the time of transplantation, but it is unclear as to what differences have led to the difference in results. Nevertheless, this study confirmed that donor–recipient size mismatch is no longer a determinant that adversely affects the long-term survival rate of transplant kidneys in pediatric KT. Thus, it is hoped that more active and safer pediatric KT can be implemented in the future.












 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















