1. Maesaka JK, Imbriano LJ, Pinkhasov A, et al. Identification of a novel natriuretic protein in patients with cerebral-renal salt wasting-implications for enhanced diagnosis.
Am J Med Sci 2021;361:261ŌĆō268.


2. Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting?
Kidney Int 2009;76:934ŌĆō938.


3. Peters JP, Welt LG, Sims EA, Orloff J, Needham J. A salt-wasting syndrome associated with cerebral disease.
Trans Assoc Am Physicians 1950;63:57ŌĆō64.

4. Maesaka JK, Imbriano L, Shirazian S, Miyawaki N. Complexity of differentiating cerebral-renal salt wasting from SIADH, emerging importance of determining fractional urate excretion. In: Vijayakumar S, Novel insights on chronic kidney disease, acute kidney injury and polycystic kidney disease. InTech; 2012. p. 41ŌĆō46.
5. Schwartz WB, Bennett W, Curelop S, Bartter FC. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone.
Am J Med 1957;23:529ŌĆō542.


6. Leaf A, Bartter FC, Santos RF, Wrong O. Evidence in man that urinary electrolyte loss induced by pitressin is a function of water retention.
J Clin Invest 1953;32:868ŌĆō878.



7. Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia.
Am J Med 1987;83:905ŌĆō908.


8. Maesaka JK, Imbriano L, Mattana J, Gallagher D, Bade N, Sharif S. Differentiating SIADH from cerebral/renal salt wasting: failure of the volume approach and need for a new approach to hyponatremia.
J Clin Med 2014;3:1373ŌĆō1385.



9. Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion.
Clin J Am Soc Nephrol 2009;4:309ŌĆō315.


10. Nelson PB, Seif SM, Maroon JC, Robinson AG. Hyponatremia in intracranial disease: perhaps not the syndrome of inappropriate secretion of antidiuretic hormone (SIADH).
J Neurosurg 1981;55:938ŌĆō941.


11. Wijdicks EF, Vermeulen M, ten Haaf JA, Hijdra A, Bakker WH, van Gijn J. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm.
Ann Neurol 1985;18:211ŌĆō216.


12. Weinman EJ, Steplock D, Suki WN, Eknoyan G. Urate reabsorption in proximal convoluted tubule of the rat kidney.
Am J Physiol 1976;231:509ŌĆō515.


13. Imbriano LJ, Mattana J, Drakakis J, Maesaka JK. Identifying different causes of hyponatremia with fractional excretion of uric acid.
Am J Med Sci 2016;352:385ŌĆō390.


14. Imbriano LJ, Ilamathi E, Ali NM, Miyawaki N, Maesaka JK. Normal fractional urate excretion identifies hyponatremic patients with reset osmostat.
J Nephrol 2012;25:833ŌĆō838.


15. Beck LH. Hypouricemia in the syndrome of inappropriate secretion of antidiuretic hormone.
N Engl J Med 1979;301:528ŌĆō530.


16. Assadi FK, John EG. Hypouricemia in neonates with syndrome of inappropriate secretion of antidiuretic hormone.
Pediatr Res 1985;19:424ŌĆō427.


17. Dorhout Mees EJ, Blom van Assendelft P, Nieuwenhuis MG. Elevation of uric aicd clearance caused by inappropriate antidiuretic hormone secretion.
Acta Med Scand 1971;189:69ŌĆō72.

18. Sonnenblick M, Rosin A. Increased uric acid clearance in the syndrome of inappropriate secretion of antidiuretic hormone.
Isr J Med Sci 1988;24:20ŌĆō23.

19. Maesaka JK, Batuman V, Yudd M, Salem M, Sved AF, Venkatesan J. Hyponatremia and hypouricemia: differentiation from SIADH.
Clin Nephrol 1990;33:174ŌĆō178.

20. Maesaka JK, Miyawaki N, Palaia T, Fishbane S, Durham JH. Renal salt wasting without cerebral disease: diagnostic value of urate determinations in hyponatremia.
Kidney Int 2007;71:822ŌĆō826.


21. Kim GH. Pathophysiology of drug-induced hyponatremia.
J Clin Med 2022;11:5810.



23. Sterns RH, Rondon-Berrios H. Cerebral salt wasting is a real cause of hyponatremia: CON.
Kidney360 2023;4:e441ŌĆōe444.


24. Cannon PJ, Svahn DS, Demartini FE. The influence of hypertonic saline infusions upon the fractional reabsorption of urate and other ions in normal and hypertensive man.
Circulation 1970;41:97ŌĆō108.


25. Steele TH. Evidence for altered renal urate reabsorption during changes in volume of the extracellular fluid.
J Lab Clin Med 1969;74:288ŌĆō299.

26. Stricker EM, Verbalis J. Water intake and body fluids. In: Squire LR, Roberts JL, Spitzer NC, Zigmond MJ, McConnell SK, Bloom FE, Fundamental neuroscience. 2nd ed. Elsevier; 2003. p. 1011ŌĆō1029.
27. Berl T. Impact of solute intake on urine flow and water excretion.
J Am Soc Nephrol 2008;19:1076ŌĆō1078.


28. Maesaka JK, Imbriano LJ, Miyawaki N. Determining fractional urate excretion rates in hyponatremic conditions and improved methods to distinguish cerebral/renal salt wasting from the syndrome of inappropriate secretion of antidiuretic hormone.
Front Med (Lausanne) 2018;5:319.



29. M├Čhring J, M├Čhring B. Reevaluation of DOCA escape phenomenon.
Am J Physiol 1972;223:1237ŌĆō1245.


30. Huang CL, Lewicki J, Johnson LK, Cogan MG. Renal mechanism of action of rat atrial natriuretic factor.
J Clin Invest 1985;75:769ŌĆō773.



31. Bussien JP, Biollaz J, Waeber B, et al. Dose-dependent effect of atrial natriuretic peptide on blood pressure, heart rate, and skin blood flow of normal volunteers.
J Cardiovasc Pharmacol 1986;8:216ŌĆō220.


32. Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone.
Am J Med 1967;42:790ŌĆō806.


33. Janicic N, Verbalis JG. Evaluation and management of hypo-osmolality in hospitalized patients.
Endocrinol Metab Clin North Am 2003;32:459ŌĆō481.


34. Verbalis JG. The curious story of cerebral salt wasting: fact or fiction?
Clin J Am Soc Nephrol 2020;15:1666ŌĆō1668.


35. Verbalis JG. Hyponatremia with intracranial disease: not often cerebral salt wasting.
J Clin Endocrinol Metab 2014;99:59ŌĆō62.



36. Sherlock M, OŌĆÖSullivan E, Agha A, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage.
Clin Endocrinol (Oxf) 2006;64:250ŌĆō254.


37. Hannon MJ, Behan LA, OŌĆÖBrien MM, et al. Hyponatremia following mild/moderate subarachnoid hemorrhage is due to SIAD and glucocorticoid deficiency and not cerebral salt wasting.
J Clin Endocrinol Metab 2014;99:291ŌĆō298.



38. Maesaka JK, Imbriano LJ. Cerebral salt wasting is a real cause of hyponatremia: PRO.
Kidney360 2023;4:e437ŌĆōe440.


39. Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful?
Ann Neurol 1985;17:137ŌĆō140.


40. Steele A, Gowrishankar M, Abrahamson S, Mazer CD, Feldman RD, Halperin ML. Postoperative hyponatremia despite near-isotonic saline infusion: a phenomenon of desalination.
Ann Intern Med 1997;126:20ŌĆō25.


41. Levinsky NG, Davidson DG, Berliner RW. Changes in urine concentration during prolonged administration of vasopressin and water.
Am J Physiol 1959;196:451ŌĆō456.


42. Sterns RH. Disorders of plasma sodium: causes, consequences, and correction.
N Engl J Med 2015;372:55ŌĆō65.


43. Corona G, Giuliani C, Parenti G, et al. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis.
PLoS One 2013;8:e80451.



44. Maesaka JK, Venkatesan J, Piccione JM, Decker R, Dreisbach AW, Wetherington J. Plasma natriuretic factor(s) in patients with intracranial disease, renal salt wasting and hyperuricosuria.
Life Sci 1993;52:1875ŌĆō1882.


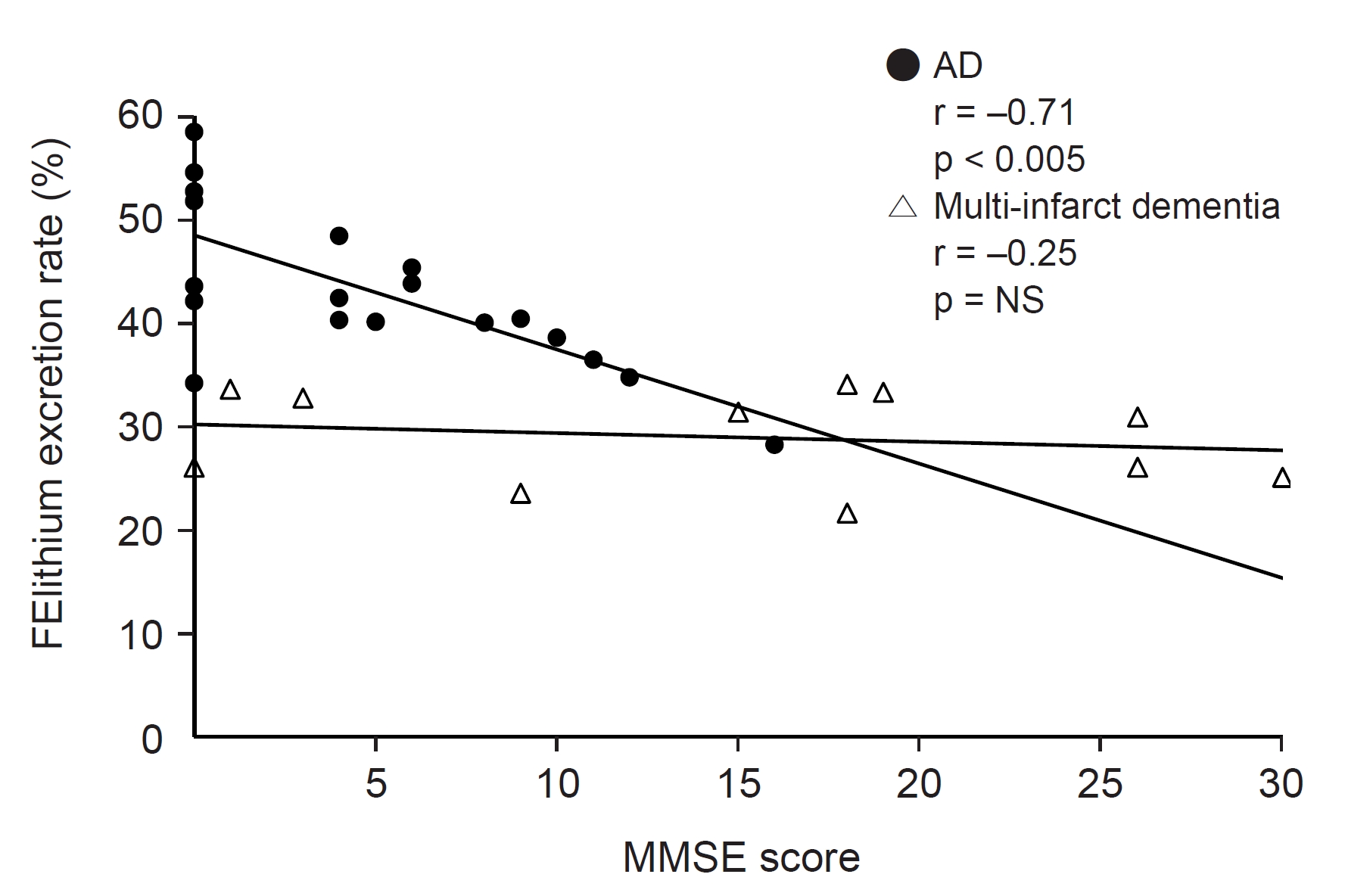
45. Maesaka JK, Wolf-Klein G, Piccione JM, Ma CM. Hypouricemia, abnormal renal tubular urate transport, and plasma natriuretic factor(s) in patients with AlzheimerŌĆÖs disease.
J Am Geriatr Soc 1993;41:501ŌĆō506.


46. Kasa M, Bierma TJ, Waterstraat F Jr, Corsaut M, Singh SP. Routine blood chemistry screen: a diagnostic aid for AlzheimerŌĆÖs disease.
Neuroepidemiology 1989;8:254ŌĆō261.


47. Hayslett JP, Kashgarian M. A micropuncture study of the renal handling of lithium.
Pflugers Arch 1979;380:159ŌĆō163.











 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















