Introduction
Ultrasound and fluoroscopy-guided punctures are recommended for safe procedures when performing catheter insertion including hemodialysis (HD) catheters. However, for unstable patients, such as those on a ventilator in an intensive care unit (ICU), transferring the patient to the fluoroscopy is subject to constraints, sometimes worsening the patient’s condition in the process [
1]. ICU patients receiving critical care often require renal replacement therapy (RRT). The insertion of an HD catheter is necessary to initiate RRT. Catheter dysfunction, which can occur during its use, can reduce the efficacy of RRT and affect patient survival, and infection complications from catheter use can lead to septicemia and can result in patient death [
2]. Compared to a non-tunneled HD catheter, a tunneled HD one has a tunnel from the exit site to the entry site into the bloodstream where the catheter is inserted. This tunnel structure is known to be effective in preventing infections. For non-tunneled HD catheters, the material is generally rather stiff for easy insertion and catheter dysfunction can occur when the catheter contacts the vessel wall during use. In contrast, the material of tunneled HD catheters is soft, and the frequency of catheter dysfunction during use is low. However, a separate instrument, such as a peel-away sheath, is required for its insertion [
3]. In brief, tunneled HD catheters are less likely to be dysfunctional, are less susceptible to infection than non-tunneled ones, and can be generally considered the first choice [
4]. However, due to the material nature of tunneled catheters, they require more attention for insertion, so it is desirable to insert them under fluoroscopic guidance, if possible. When the patient’s condition is unstable, we often hesitate to insert a tunneled HD catheter without fluoroscopic guidance.
In this study, we aimed to demonstrate the safety and durable patency of tunneled HD catheter insertion without fluoroscopic guidance in our institution by retrospectively comparing cases without fluoroscopy to those conducted with fluoroscopy.
Methods
The study protocol was approved by the Institutional Review Board of Eunpyeong St. Mary’s Hospital (No. PC22RASI0204), and all patients provided written informed consent. The study population was initially comprised of 1,157 consecutive cases that underwent HD catheter insertion between June 2019 and September 2022 from the HD catheter cohort at Eunpyeong St. Mary’s Hospital. We excluded cases with only acute femoral catheter use (n = 273). Those with acute jugular catheter use (n = 181), an over-the-guidewire exchange from a former acute jugular catheter to a newly tunneled jugular one (n = 53), and an over-the-guidewire exchange from a former jugular tunneled catheter to a newly tunneled jugular one (n = 66) were then excluded. De novo femoral tunneled catheter placements (n = 5) and femoral tunneled cases by the over-the-guidewire method (n = 3) were also excluded. We do not perform left-sided tunneled HD catheter insertion without fluoroscopy in our institution because the left brachiocephalic vein is tortuous, and the procedure can be associated with serious vessel damage or perforation without fluoroscopy. Therefore, left-sided tunneled HD catheter insertion cases (n = 21) were also excluded from the comparison. Finally, 81 cases of tunneled HD catheter insertion without fluoroscopic guidance and the other 474 cases of tunneled HD insertion with fluoroscopy were compared and analyzed in this study.
Catheter insertion techniques
The catheter insertion procedures were performed by either an interventional nephrologist or radiologist staff. Fresh frozen plasma was used in the cases with prothrombin time (PT) or activated partial thromboplastin time (aPTT) prolongation by clinician’s discretion, whereas we did not use pre-treatments to minimize uremic bleeding such as Desmopressin diacetate arginine vasopressin (DDAVP) or cryoprecipitate. The patients were sedated with intravenous midazolam and fentanyl at the interventionalist’s discretion. In fluoroscopic guidance insertion, tunneled HD catheters were placed under both ultrasound and fluoroscopic guidance in patients who were able to move to the angiography suite.
In the cases of tunneled HD catheter insertion without fluoroscopy, tunneled HD catheters were placed with only ultrasound guidance for vascular punctures in patients who were unable to move to the angiography suite, commonly in the ICU. In such a scenario, initially in the former cases (about 30 to 40 cases), the appropriate catheter length site was estimated based on the patient’s height, as well as measurements on the patient’s recent chest X-ray. In the later 50 to 60 cases, we used the manubrial-sternal angle (angle of Louis) as a topographical landmark for the carina [
1,
5]. The estimated insertion depth was selected by adding the distance between the puncture site and a point 5 cm below the manubrial-sternal angle. There was no difference in the development rate of catheter dysfunction between these two measurement methods. However, when measured by the latter method, the catheter tip is mostly located at a little bit higher than the former one.
We also routinely used a 0.035-inch hydrophilic straight guidewire rather than a J-tip guidewire, which was originally prepared within the catheter set, because hydrophilic guidewires rarely kink. First, under ultrasonographic guidance, the targeted internal jugular vein was punctured with a 21-gauge needle rather than an 18-gauge, which was originally prepared within the catheter set, because a 21-gauge needle is less traumatic even in the case of an arterial puncture. After confirming good venous return, a 0.018-inch hairy guidewire was inserted, and a 4 French (F) or 5F coaxial sheath was placed. The introduction of this hairy guidewire is expected to be very smooth. If there was any abnormal resistance while downing the hairy guidewire, the wire was repositioned. If any abnormal resistance remained even after repositioning, a new puncture was sometimes made. There were eight cases where the tunneled HD catheter insertion without fluoroscopy was impossible because of the disability of downing a hairy guidewire. Therefore, the failure rate of HD catheter insertion into a right internal jugular vein without fluoroscopy was 9% (8 of 90).
In such cases, femoral non-tunneled catheters were placed under ultrasonographic guidance. The right internal jugular vein was identified in ultrasound examination in all eight cases. In two cases, right innominate vein occlusion was found in angiography later so left-sided HD catheter insertion was fulfilled under fluoroscopy. In the other four cases, subsequent angiogram showed the right innominate vein was tortuous making downing a hairy guidewire without fluoroscopy difficult. In the last two cases, the patients died before considering a new HD catheter insertion.
Next, the coaxial sheath was introduced, and the 0.035-inch hydrophilic straight guidewire was inserted next through this coaxial outer sheath under continuous cardiac monitoring to detect cardiac arrhythmia, which indicated the wire tip in the right atrium. However, because fluoroscopy was unavailable in this situation, more attention was paid to not placing the guidewire too deep within the right atrium. The venotomy site was sequentially dilated using two dilators provided in the kit. A small incision was created as an exit site, and a subcutaneous tunnel was created next. Peeling away the outer sheath combined with inner-third dilator insertion was the most attentive step in the tunneled HD catheter insertion without fluoroscopy. We did not insert the third dilator with a peel-away sheath to its full length. After a characteristic “pop” was perceived by the interventionalist when the third dilator with a peel-away sheath was inserted into the internal jugular vein, the third dilator with a peel-away sheath was inserted to 3/4 or 4/5 of its full length, leaving the distal portion (1/4 or 1/5) at the venotomy site. The third inner dilator was withdrawn, and the tunneled HD catheter was introduced into the peel-away outer sheath. The catheter was advanced by gradually peeling away the outer sheath. In addition, the peel-away outer sheath was not advanced further after retracting the third inner dilator because the tip of the separated outer sheath could damage the vessel wall. Catheter function was checked by rapidly aspirating blood with a 3-mL locking syringe to see if there was resistance or a characteristic “tuck.” A post-procedural chest X-ray was obtained to confirm the catheter configuration and distal tip location. The tunneled catheters used were Glidepath (Bard) or Palindrome (Covidien).
Definitions
Technical success of the tunneled HD catheter insertion was defined as the completion of at least one HD session or 24-hour continuous RRT with an adequate flow rate. The incidence of immediate complications (within 1 day of catheter insertion), such as prolonged bleeding requiring additional suturing after the procedure or hematoma, and long-term or non-immediate complications, such as infection or catheter dysfunction, were compared between the two groups. Catheter dysfunction was defined as the insufficient maintenance of the blood flow rate due to thrombi or fibrin sheath formation. Catheter patency was calculated from the insertion of the tunneled catheter until the catheter removal due to its dysfunction or infection or the final follow-up date before data collection for this study began. However, when the functional catheter was removed because the patient’s arteriovenous fistula or graft was matured or the patient died, such catheter removals were analyzed as censored data. Catheter infections requiring catheter removal included catheter-related bacteremia, resistant exit infections, and tunnel infections. In the electronic medical records, catheter-related bacteremia was regarded as positive blood cultures obtained from the catheter of a febrile patient without any other source of infection. Exit-site infection was regarded as the presence of a discharge from the exit or soreness without any tenderness over the tunnel, whereas a tunnel infection was regarded as not only the presence of a discharge from the exit or erythema but also tenderness or induration over the tunnel itself, regardless of whether the discharge yielded a positive culture.
Data collection
The demographic data collected included age, sex, cause of catheter insertion (acute kidney injury [AKI] vs. end-stage renal disease [ESRD]), and the patient status (ICU or ward). The closest laboratory data to the point of a tunneled HD catheter insertion collected included white blood cell (WBC) count, hemoglobin, platelet count, blood urea nitrogen, serum creatinine (sCr), PT, aPTT, C-reactive protein (CRP), and serum albumin level. Almost all the laboratory samples were collected on the morning of a tunneled HD catheter insertion or the day before an indexed procedure.
Statistical analysis
The results for continuous variables with a normal distribution are presented as the mean ± standard deviation, and the results for variables without a normal distribution are presented as the median and interquartile range. The Student t test or the Mann-Whitney U test was used, as appropriate, to determine the significance of differences in the continuous variables between groups. Categorical variables are presented as percentages. Pearson chi-square test or Fisher exact test was used for determining the significance of differences in the categorical variables between groups. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Cox proportional-hazard regression models were used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) for catheter patency. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS version 23.0 for Windows (IBM Corp.).
Results
Diabetes mellitus was more prevalent in the group with fluoroscopy compared to the group without fluoroscopy. There were more AKI and ICU patients in the group without fluoroscopy. Such group characteristics without fluoroscopy were also compatible with higher WBC counts, lower platelet counts, more prolonged PT, and more prolonged aPTT in the group without fluoroscopy. Similarly, CRP levels were also higher in the group without fluoroscopy. In contrast, sCr levels were lower in the group without fluoroscopy, consistent with the current real clinical practice where early RRT is commonly performed in AKI patients while early RRT is no longer recommended for ESRD patients (
Table 1).
Between the two groups, immediate complications after catheter insertion and long-term complications requiring catheter replacement including dysfunction, infection, catheter slippage, and breakage, were comparable (
Table 2).
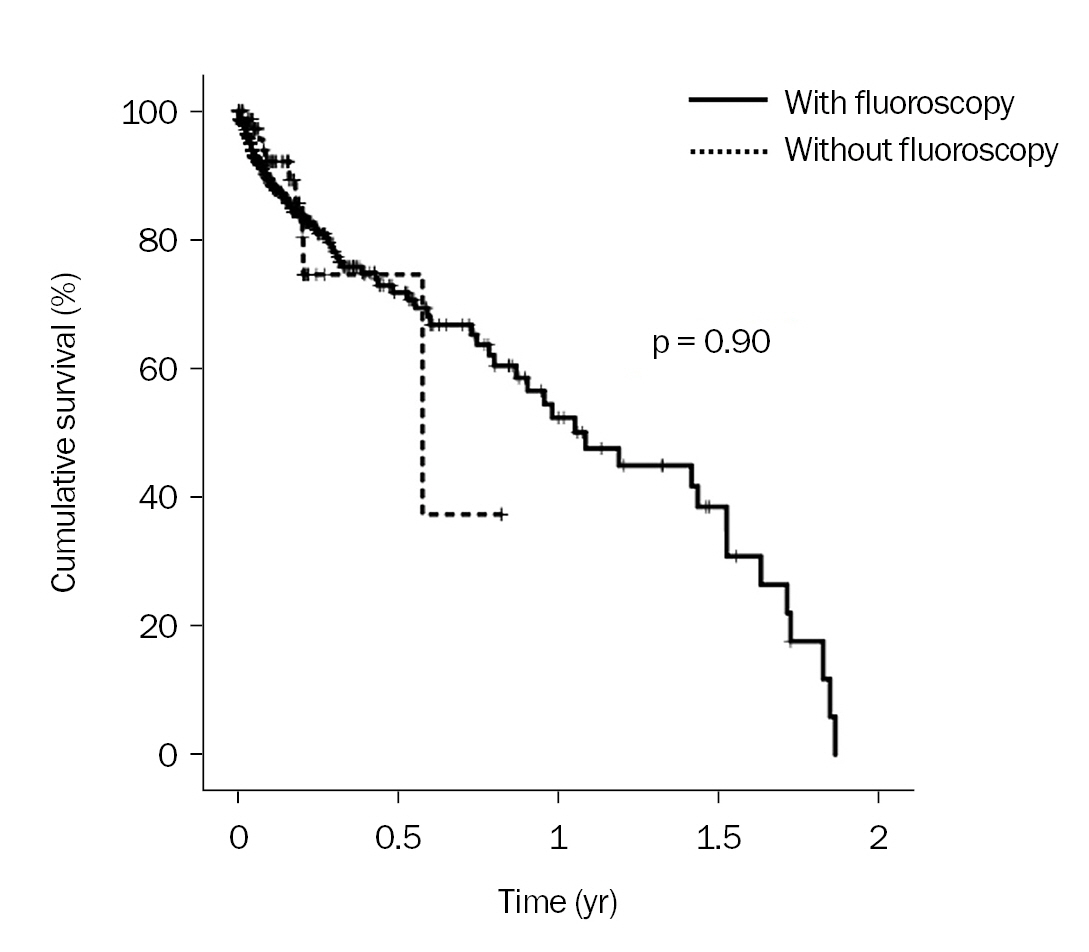
The patency of tunneled catheters inserted without fluoroscopy was also comparable to that of tunneled catheters inserted with fluoroscopy (p = 0.90) (
Fig. 1). When we compared among ICU patients only, we found that the two methods were still similar (p = 0.70) (
Fig. 2A). Likewise, the two methods were also comparable among ward patients (p = 0.61) (
Fig. 2B). Univariate and multivariate Cox regression analyses were performed to assess the effects of variables on the catheter patency, in which the potential confounders were included (
Table 3). In the multivariate Cox regression analysis of catheter patency, tunneled catheter placement without fluoroscopy (vs. with fluoroscopy) also did not influence catheter patency (HR, 0.96; 95% CI, 0.44–2.11; p = 0.92).
Discussion
The new 2019 Kidney Disease Outcomes Quality Initiative (KDOQI) vascular guideline recommended that tunneled HD should be inserted under imaging guidance using both ultrasound and fluoroscopy as in the former KDOQI 2006 guideline for safety and correct catheter tip positioning [
4,
6]. However, it is not always easy to use fluoroscopy when needed if a hospital does not have its own nephrology angiography suite. Several studies previously reported the safety and comparable patency of tunneled HD catheter insertion or exchange from non-tunneled without fluoroscopy [
7–
12]. More recently, some authors reported the additional benefits of tunneled HD catheter insertion without fluoroscopy from the perspective of coronavirus 2019 (COVID-19) infection prevention [
1,
13]. Those suggested that an isolated catheter insertion procedure within the patient’s ICU room without fluoroscopy could minimize COVID-19 exposure in hospital personnel and the waste of available hospital resources. Some of those previous studies reported only the safety and technical success rate of tunneled HD catheter without fluoroscopy and did not compare it to a group with fluoroscopy [
1,
9–
13]. However, a few of those studies demonstrated the safety and comparable patency of tunneled HD catheter insertion without fluoroscopy compared to a group with fluoroscopic guidance during the same period, as was analyzed in our study [
7,
8].
For a long time, non-tunneled HD catheters with or without ultrasound guidance have been inserted in the ICU by nephrologists or critical care physicians. The choice between non-tunneled and tunneled HD catheters remains unresolved, considering the limited life expectancy of ICU AKI patients [
5,
14]. However, a non-tunneled HD catheter has definite weak points compared to a tunneled one. Basically, it is designed for convenience in bedside insertion. Its relative stiffness, sometimes similar to a dilator within a catheter kit, can ease the insertion procedure via a guidewire. However, such stiffness may cause dysfunction during use when a non-tunneled catheter contacts a vessel wall. A stiff non-tunneled HD catheter remains stuck at the vessel wall without slipping. In addition, the vessel entry point is directly open to the skin with a non-tunneled HD catheter, so the infection risk is higher compared to a tunneled one, where a vessel entry point is covered with instantly sutured skin or completely healed skin later. In contrast to a non-tunneled HD catheter, a tunneled HD catheter is created to be soft to prevent its dysfunction. When not connected to a dialysis machine, it is never stuck when it touches the vessel wall. When connected, even when it shows a tendency to approach the vessel wall due to the negative pressure of the aspirating arterial side, such a tendency is minimized because of its softness. But this soft tunneled HD catheter can also become stuck to the vessel wall if in conditions of high negative pressure. The recently developed symmetric tip HD catheter has the advantage of switching the arterial and venous lumens when the arterial lumen is stuck to the vessel wall, working well without significant blood recirculation [
15].
Paradoxically, the softness of tunneled HD catheters can be an obstacle during their initial insertion, so a peel-away sheath with a third dilator is equipped within the catheter kit to facilitate soft tunneled catheter insertion. The rigid and pointed third dilator can penetrate a vessel wall, so its position should be constantly monitored under fluoroscopy if available. Even without fluoroscopic guidance, this third dilator can be advanced but should not be advanced too deeply so that it reaches the right atrium. For this, in our institution, the third dilator with a peel-away sheath is inserted to 3/4 or 4/5 considering its full length, leaving the distal portion (1/4 or 1/5 of it) at the venotomy site.
In our institution, we do not attempt to insert an HD catheter into the left internal jugular vein, either tunneled or non-tunneled, although central venous catheterization is performed using the left internal jugular vein. This is because the left brachiocephalic vein is not straight but tortuous, unlike the right brachiocephalic vein. A large-bore dilator within the HD catheter kit without fluoroscopic guidance can perforate a vessel wall if the left brachiocephalic vein is very tortuous or at an acute angle. Some serious consequences have been reported [
16–
18]. That is why our current study did not include left-side inserted tunneled catheters.
The interventional nephrologist in our institution who inserted tunneled HD catheters without fluoroscopy already had performed more than a thousand tunneled HD catheter insertions with fluoroscopy, so he is a very skillful interventionalist. Therefore, our study results do not simply imply that tunneled HD catheter insertion without fluoroscopy by any medical personnel is both safe and comparable to the formal method with fluoroscopic guidance. However, because many nephrologists already are used to acute HD catheter blind insertion, we think any nephrologist, who has only additional interests in HD catheter insertion using ultrasound, can perform this tunneled HD catheter insertion without fluoroscopy, especially in ICU after education and sufficient practice.
However, based on our study results, tunneled catheter insertion without fluoroscopy can be positively considered and then performed more frequently than it is currently, especially in ICU patients.













 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















