Clinical relevance of blood urea nitrogen to serum albumin ratio for predicting bacteremia in very young children with febrile urinary tract infection
Article information
Abstract
Background
Urinary tract infections (UTIs) are one of the most common bacterial infections in febrile children and a common cause of hospitalization, especially in very young children. We examined the clinical characteristics and predictive factors of concomitant bacteremia in pediatric patients with febrile UTI aged ≤24 months.
Methods
This retrospective multicenter study reviewed medical data from 2,141 patients from three centers from January 2000 to December 2019. Enrolled cases were classified into the bacteremic UTI and non-bacteremic UTI groups according to the presence of blood culture pathogens.
Results
Among 2,141 patients with febrile UTI, 40 (1.9%) had concomitant bacteremia. All patients in the bacterial group were aged ≤6 months. Multivariate analysis revealed that younger age, lower blood lymphocyte counts and serum albumin levels, higher C-reactive protein (CRP) levels, blood urea nitrogen (BUN) levels, and BUN/serum albumin ratio were independent risk factors of concomitant bacteremia. The area under the receiver-operating characteristic curves predicting bacteremia were 0.668 for CRP, 0.673 for lymphocytes, and 0.759 for the BUN/albumin ratio.
Conclusion
The present study identified the BUN/albumin ratio and lower blood lymphocyte counts as novel predictive factors for bacteremia in young infants with febrile UTI in addition to the previously identified factors of younger age and higher CRP levels. Our findings could help to identify patients at high risk of bacteremia and benefit decision-making in the management of infants with febrile UTI.
Introduction
Urinary tract infections (UTIs) are one of the most common bacterial infections in febrile children and a common cause of hospitalization, especially in very young children [1–3]. Most patients with febrile UTI have an uncomplicated clinical course [4]; however, some experience complications, including bacteremia, during or as a result of febrile UTI. UTI with bacteremia is associated with poor outcomes, such as longer hospitalization, intensive care unit (ICU) admission, severe sepsis, and meningitis [5,6]. Since younger age is a well-known risk factor for bacteremia [7–9], clinicians routinely hospitalize very young infants with UTI for intravenous antibiotic therapy due to concerns about bacteremia. However, recent studies reported that outpatient treatment with oral antibiotics is safe and cost-saving for young infants with febrile UTIs unless the patient is at high risk of bacteremia [10,11]. Therefore, it is helpful to predict concurrent bacteremia in patients with febrile UTI prior to culture test to avoid unnecessary hospitalization of low-risk patients and initiate the management of high-risk patients. Previous studies have reported predictive factors for bacteremia in febrile children with UTI in terms of clinical presentation (e.g., ill appearance and poor feeding), laboratory findings (e.g., increased serum creatinine levels or inflammatory markers, such as C-reactive protein [CRP], and procalcitonin), and abnormalities in imaging studies [5,7,12,13]. However, the characteristics of the patient cohorts, including age, severity of patients’ illness, and number of participants, varied, and related findings for bacteremia were not consistent between studies. The present study examined the clinical and laboratory risk factors for concomitant bacteremia among 2,141 children aged ≤24 months with febrile UTI at initial presentation to confirm previously established factors and identify novel findings.
Methods
This study received ethical approval from the Institutional Review Board (IRB) of The Catholic University of Korea, Bucheon St. Mary’s Hospital (No. XC20RIDI0046). As the study subjects were deidentified, the IRB waived the need for written consent from the patients.
Data collection
This multicenter retrospective study was performed at three university hospitals and included very young children aged ≤24 months with UTI who were initially admitted presenting with fever (≥38.0 ℃) from January 2000 to December 2019. Medical records were reviewed to obtain information on the patients’ clinical characteristics, including sex, age at admission, history of previous hospitalization and use of antibiotics, initial laboratory findings, and results of urologic images, including kidney ultrasonography (USG), 99m-Tc dimercaptosuccinic acid (DMSA) renal scan, and voiding cystourethrography (VCUG).
Laboratory findings
UTI was defined as a single pathogen titer of >100,000 colony-forming units/mL from a sterile bag-collected specimen or >50,000 colonies from a catheterized specimen from a patient with fever [14,15]. Blood cultures were obtained for all patients. Bacteremic UTI was defined as a UTI of a single pathogen in both blood and urine cultures. Patients were excluded if: their medical records or data were insufficient; their fever was caused by another condition, such as pneumonia, neutropenic fever, or malignancy; the same bacteria were not detected in blood and urine cultures; or they received ICU care. Degree of pyuria (≥5 white blood cells [WBC] per high power field [HPF]) in urine microscopy was categorized as: <5 WBC/HPF (no pyuria); 5 to 49 WBC/HPF; and ≥50 WBC/HPF. Since the reference ranges for the erythrocyte sedimentation rate (ESR) and CRP levels differed at each center, they were expressed as a multiple of the upper limit of the normal value for each institution. The neutrophil-to-lymphocyte (NLR) and platelet-to-lymphocyte (PLR) ratios have been reported as systemic inflammatory markers and predictive factors for bacteremia [16–18] and are calculated using a complete blood count. Blood urea nitrogen (BUN) and serum albumin levels were measured and expressed as a ratio (BUN/albumin) [19]. Abnormal kidney USG included hydronephrosis, obstructive uropathy, duplex kidney, and cystic kidney disease. A focal reduction or absence of uptake in more than one area of the kidney was considered abnormal in DMSA renal scan.
Statistical analysis
Enrolled cases were classified as bacteremic UTI and non-bacteremic UTI groups according to the presence of blood culture pathogens, and clinical and laboratory data were compared between the two groups. The chi-square test or Fisher exact test was used for categorical variables and t test or Mann-Whitney test was used for continuous variables. Univariate and multivariate analyses were performed using logistic regression to identify significant risk factors for bacteremic UTI. Variables significantly associated with bacteremia in the univariate analysis were included in the multivariate analysis. Receiver-operating characteristic (ROC) curve analysis was performed by estimating the area under the curve (AUC) to identify the optimum cutoff values of variables that were significantly associated with bacteremia in multivariate analysis for the prediction of bacteremia. The maximum value of the Youden index was selected as the optimal cutoff value. The p-values of <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 22.0 (IBM Corp.).
Results
Clinical characteristics
A total of 2,141 patients aged ≤24 months with febrile UTI were identified during the study period. Table 1 describes the demographics of the patients. Among these patients, 40 (1.9%) were in the bacteremic group and 2,101 (98.1%) were in the non-bacteremic group. The mean age of all the patients was 4.94 months and 43.8% were aged 0 to 3 months, 34.5% were 4 to 6 months, 17.1% were 7 to 12 months, and 4.7% were 13 to 24 months. The mean age of the bacteremic group was younger than that of the non-bacteremic group (3.05 ± 1.68 months vs. 4.98 ± 3.76 months, p < 0.001). None of the patients in the bacteremic UTI group were aged ≥7 months. Among the patients in the bacteremic group, 29 (72.5%) were aged 0 to 3 months and 11 (27.5%) were 4 to 6 months. In contrast, among patients in the non-bacteremic group, 908 (43.2%) were aged 0 to 3 months and 727 (34.6%) were 4 to 6 months. Among all patients, 68.7% were male and the mean duration of fever before admission was 1.6 days. Sex distribution and duration of preceding fever before admission did not differ between the bacteremic and non-bacteremic groups. Among them, 117 (5.47%) and 128 (5.98%) had a history of hospitalization and received antibiotics therapy in the previous 3 months, respectively. Three patients in the bacteremic group had been hospitalized within 3 months before UTI admission due to feeding problems with laryngomalacia, respiratory viral infection, and rotaviral enteritis, respectively. In addition, one patient with a respiratory viral infection received empirical antibiotics during hospitalization. The proportions of patients with a history of hospitalization or antibiotics therapy before admission also did not differ between the two groups.
Laboratory and imaging findings
Table 2 shows comparisons of the laboratory and imaging findings between the two groups. Pyuria (≥5 WBC/HPF) was present in 2,002 patients (93.5%) and absent in 139 patients (6.5%). Escherichia coli was the most frequently isolated bacterium in both the bacteremic and non-bacteremic UTI groups (97.5% and 87.7%, respectively). Among the patients who had E. coli or Klebsiella spp. in their urine culture, extended-spectrum β-lactamase (ESBL)-positive bacteria were found in six patients (15.0%) in the bacteremic UTI group and 326 (15.5%) in the non-bacteremic UTI group, respectively. There were no differences between the bacteremic and non-bacteremic UTI groups in terms of the degree of pyuria, types of isolated bacteria, or proportion of ESBL positivity.
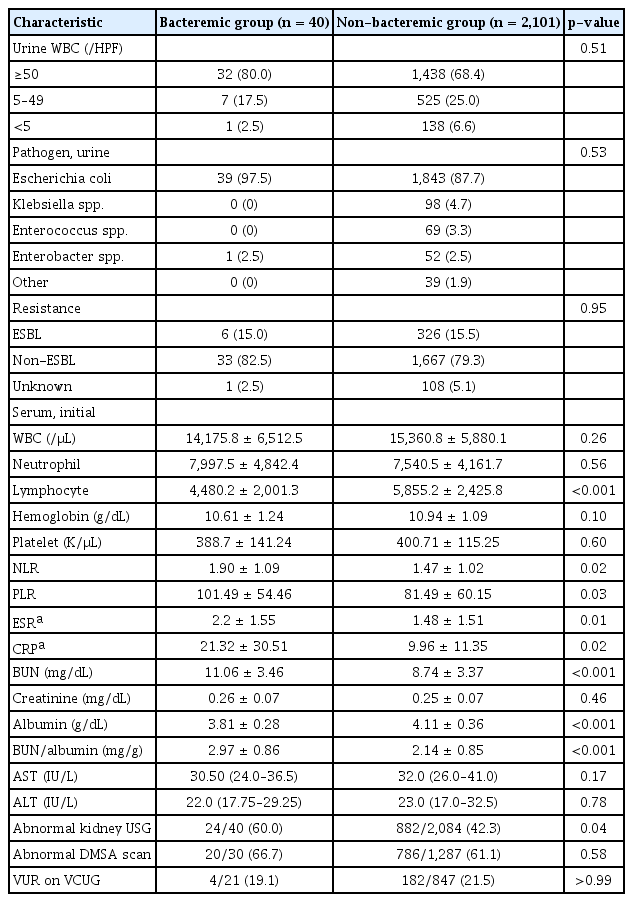
Laboratory and imaging findings of patients with bacteremic and non-bacteremic urinary tract infection
On admission, there were no significant differences in WBC, neutrophil, hemoglobin, or platelet counts between the bacteremic and non-bacteremic UTI groups. However, NLR (p = 0.02), PLR (p = 0.03), ESR (p = 0.01), and CRP levels (p = 0.02) were significantly elevated and lymphocyte levels (p < 0.001) were decreased in patients in the bacteremic group. Serum creatinine, aspartate transaminase, and alanine transaminase levels did not differ between the two groups. However, BUN levels were elevated and albumin levels decreased (p < 0.001 for both), subsequently the BUN/albumin ratio was higher in the bacteremic group (p < 0.001).
Kidney USG was available in 40 patients (100%) in the bacteremic group and 2,084 (99.2%) in the non-bacteremic group. Abnormal USG findings, such as hydronephrosis or pyelonephritis, were more frequent in the bacteremic group than in the non-bacteremic group (60.0% in the bacteremic group vs. 42.3% in the non-bacteremic UTI group; p = 0.04). However, the proportions of abnormal findings on DMSA scan (66.7% in the bacteremic group vs. 61.07% in the non-bacteremic group) and VCUG (19.1% in the bacteremic group vs. 21.49% in the non-bacteremic group) did not significantly differ between two groups.
Factors associated with bacteremic urinary tract infection
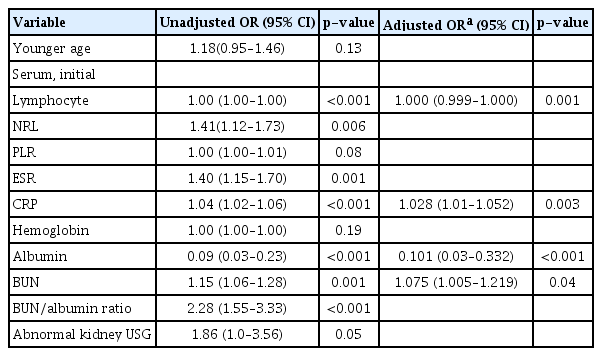
Univariate analysis revealed that younger age, lower lymphocyte and serum albumin levels, higher NRL, ESR, CRP, BUN levels and BUN/albumin ratio, and abnormal kidney USG findings were significantly associated with increased risk of bacteremic UTI (Table 3). Multivariate analysis showed that younger age (odds ratio [OR], 1.31; 95% confidence interval [CI], 1.12–1.70; p < 0.001), lower lymphocyte levels (OR, 1.00; 95% CI, 1.00–1.00; p = 0.005), higher CRP levels (OR, 1.03; 95% CI, 1.01–1.05; p = 0.005), lower albumin levels (OR, 0.22; 95% CI, 0.07–0.71; p = 0.01), and higher BUN levels (OR, 1.07; 95% CI, 1.00–1.18; p = 0.04) were independent risk factors associated with the development of bacteremia in patients with febrile UTI. Multivariate analysis revealed that younger age was an independent risk factor for the development of bacteremia. Moreover, a significant age difference was observed between the bacteremic and non-bacteremic groups. In addition, univariate and multivariate logistic regression analyses were performed on participants aged 6 months or younger (n = 40 for the bacteremic group and n = 1,635 for the non-bacteremic group) to confirm whether the four laboratory variables (lower lymphocyte levels, higher CRP levels, lower albumin levels, and higher BUN levels) were independent risk factors associated with the development of bacteremia regardless of age (Table 4). In the multivariate analysis, lower lymphocyte (OR, 1.00; 95% CI, 1.00–1.00; p = 0.001), higher CRP levels (OR, 1.03; 95% CI, 1.01–1.05; p = 0.003), lower albumin levels (OR, 0.10; 95% CI, 0.03–0.33; p < 0.001) and higher BUN levels (OR, 1.08; 95% CI, 1.01–1.22; p = 0.04) were independent risk factors for bacteremia in febrile UTI patients aged 6 months or younger.

Univariate and multivariate analyses of risk factors associated with bacteremic UTI in patients aged ≤24 months
Cutoff values for laboratory variables predicting bacteremic urinary tract infection
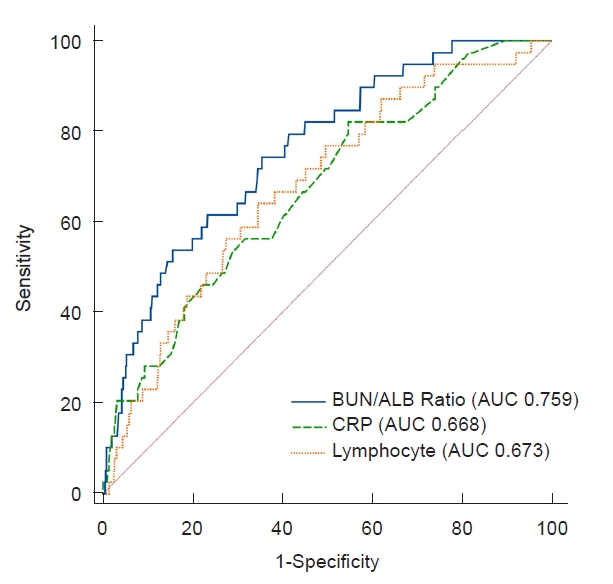
ROC analysis was performed for laboratory variables that were found to be independent risk factors for bacteremia in the multivariate analysis (Fig. 1). The AUCs were 0.67 (95% CI, 0.59–0.76; p < 0.001) for lymphocytes, 0.67 (95% CI, 0.59–0.75; p < 0.001) for CRP, and 0.76 (95% CI, 0.69–0.83; p < 0.001) for the BUN/albumin ratio. The optimal cutoff value for the BUN/albumin ratio for predicting bacteremia was 2.39, with 74.4% sensitivity and 64.2% specificity. The optimal values of lymphocyte count and serum CRP level were 4,772.25/μL with 62.5% sensitivity and 65.4% specificity, and 5.32 (5.32 times the upper limit of the normal value) with 82.5% sensitivity and 45.1% specificity, respectively.
Discussion
The present multicenter study revealed the clinical characteristics and risk factors for febrile UTI with concomitant bacteremia in very young children. Our study identified 40 patients (1.9%) aged ≤24 months with febrile UTIs with concomitant bacteremia. The incidence of bacteremia was 3.11% (29 of 937) in children aged 0 to 3 months and 1.5% (11 of 738) in those aged 4 to 6 months. There were no differences based on sex. Previous studies have reported various incidence rates of bacteremic UTI between 0% and 21%, depending on the age group studied and study design, and most studies have reported younger age as a risk factor [6–8,20]. This finding was also consistent with the findings of our study. Bacteremic UTI only developed in children aged ≤6 months and was inversely related to age.
As previously reported, higher CRP levels were also associated with bacteremic UTI in the present study [7,12,20]. While initial WBC or neutrophil counts and degree of urine pyuria are unable to distinguish patients with bacteremic UTI from those with non-bacteremic UTI, the mean CRP level in patients in the bacteremic UTI group was around twofold higher than that of those in the non-bacteremic UTI group and 21 times higher than the upper normal value. However, the CRP levels observed in the bacteremic and non-bacteremic groups overlapped considerably, as previously reported [12]. This was expressed as a relatively low specificity of CRP level in ROC analysis for predicting bacteremia. In addition, the cutoff value predicting bacteremia varied between studies [7,20,21]. Therefore, high CRP levels could be useful to predict bacteremic UTI, although CRP levels alone may not be sufficient and other clinical and laboratory factors should also be considered.
This study revealed that lower lymphocyte level was also an independent risk factor for bacteremia. Previous studies have shown that low lymphocyte counts are associated with poor prognosis in acute and chronic inflammatory conditions such as sepsis, cancer, and cardiovascular and pulmonary diseases [22–24]. However, it has not yet fully understood how low lymphocyte causes immune system abnormalities in patients with pediatric UTI and increases bacteremia [25,26]. Therefore, further research is needed on these mechanisms and cutoff values of lymphocyte levels to predict bacteremia in young infants with febrile UTI.
We found that higher levels of BUN and lower serum albumin levels were also independent risk factors for bacteremia. Based on the findings that patients with bacteremia had elevated levels of BUN but not serum creatinine, these patients appeared to be more dehydrated than non-bacteremic patients during severe illness due to reduced kidney function compared with non-bacteremic patients [27]. Lower levels of albumin result from the combined effects of decreased synthesis during the acute phase of inflammation and capillary leakage of albumin due to endotoxins released from Gram-negative bacteria, cytokines (e.g., interleukin 6), and chemokines [28]. In a study of adult patients with UTI, Leibovici et al. [29] reported lower albumin levels were an independent predictor of bacteremia. In addition, a recent study on febrile infants with UTI reported that serum albumin levels were lower in bacteremic UTI than in non-bacteremic UTI, although serum albumin levels were not associated with bacteremia in the multivariate analysis [7].
In the present study, the combined use of two parameters, elevated BUN and lower serum albumin (expressed as a ratio), showed the highest AUC (0.76 [95% CI, 0.74–0.78]; p < 0.001) in the ROC analysis, with an optimum cutoff value of 2.39 (mg/g). Several recent studies have highlighted the serum BUN/albumin ratio as a novel prognostic parameter predicting disease severity or mortality in adult patients with various inflammatory conditions [28,30,31]. Ryu et al. [27] reported that a BUN/albumin ratio of >7 mg/g at the initial visit to the emergency room was associated with increased mortality within 28 days in patients with aspiration pneumonia. In a study on patients with coronavirus disease 2019, elevated BUN/albumin ratio at admission was found to be an independent risk factor for critical outcomes, including admission to ICU, requirement for mechanical ventilation, or death [30]. In addition, Zou et al. [25] reported that an increased BUN to albumin ratio was a significant predictor of higher mortality rate and ICU requirement at the onset of E. coli bacteremia. To the best of our knowledge, the association between serum BUN/albumin ratio and UTI has not been previously reported in pediatric patients. However, based on the present study, BUN/albumin ratio could be a useful prognostic parameter for predicting bacteremia in young children with febrile UTI, though further validation is required to clarify its association with bacteremia in febrile UTI.
Genitourinary (GU) tract anomaly is associated with bacteremic UTI in children [7,12]. Yoon et al. [7] reported that VUR was found nearly twofold in bacteremic UTI infants than in those with non-bacteremic UTI (59.3% vs. 30.6%). Moreover, the presence of VUR was associated with the development of bacteremia in multivariate logistic regression analysis (OR, 3.66; 95% CI, 1.33–10.05; p = 0.012). Similarly, a study in Finnish children with bacteremic UTI showed that urinary tract obstruction (9% vs. 1%, p < 0.01) and grade 3 to 5 VUR (30% vs. 16%, p = 0.02) were detected more frequently in bacteremic patients with UTI than those without bacteremia [12]. In this study, abnormal USG findings such as hydronephrosis or pyelonephritis were associated with bacteremic UTI in univariate regression analysis. However, such an association was not observed in multivariate analysis. Although the relationship between GU tract anomaly and bacteremic UTI remains to be determined entirely, we suggest clinicians should consider imaging studies to evaluate the presence of GU tract anomaly in pediatric patients with bacteremic UTI, as suggested in previous studies [7,12].
UTI is the most common primary source of Gram-negative infection in the blood of children. Infants aged <1 year were found to have the highest incidence of Gram-negative bloodstream infection among children and younger age was associated with mortality [32]. Therefore, early identification of bacteremia in infants with febrile UTI may reduce complications via prompt management, close monitoring of hemodynamic state, and early transfer to the ICU when indicated [13]. In contrast, outpatient and oral management with appropriate follow-up is safe, has lower medical costs, and prevents potential iatrogenic complications associated with hospitalization for young infants with UTI at low risk for bacteremia [10]. Ill appearance at initial presentation and/or elevated inflammatory markers, such as WBC count and CRP levels, can help predict patients’ risk of adverse events. However, many young infants with bacteremia show nonspecific symptoms at initial presentation and clinical judgment depends on the personal experience of clinicians [33]. The present study revealed that elevated BUN/albumin ratio, which is simple and easy to calculate and is not affected by the treating physician’s subjectivity, as well as higher CRP levels and lower blood lymphocyte counts may help to predict the risk of bacteremia in very young infants with febrile UTI [25].
The present study has some limitations. This was a retrospective cross-sectional study; therefore, some data, such as recurrence of UTI or follow-up laboratory data, were unavailable. Furthermore, only 40.5% (868 of 2,141) of the children in the present study underwent VCUG. As a result, there may be a statistical bias on the results of VUR and bacteremia in the present study. However, this was because we followed the recommendations of the American Academy of pediatrics guidelines, which do not recommend performing VCUG routinely after the first febrile UTI in children aged <24 months due to its risk of exposure to radiation and invasiveness [34]. Nevertheless, a strength of our study was that it was a multicenter study and the population size was relatively large considering the pediatric focus of the study.
In conclusion, the present study examined clinical and laboratory parameters to identify risk factors for bacteremia in pediatric UTI patients aged ≤24 months. Younger age, higher CRP levels, lower blood lymphocytes, and elevated BUN/albumin ratio were significant risk factors for bacteremia in febrile UTI. Our findings could be helpful to identify patients at high risk of bacteremia and enable appropriate decision-making in the management of febrile infants with UTI. Further prospective studies are required to validate the diagnostic value and establish reliable cutoff values for these parameters.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This study was supported by the Institute of Clinical Medicine Research of The Catholic University of Korea, Bucheon St. Mary’s Hospital Research Fund (BCMC20BD04).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization, Data curation: HH, YL, JSS
Formal analysis: NYK
Methodology: YL, NYK
Project administration: JSS
Writing–original draft: HH, YL, JSS
Writing–review & editing: YL, JSS
All authors read and approved the final manuscript.
Acknowledgements
Statistical analysis was supported by biostatisticians employed by CC&I Research Co., Ltd.