| Kidney Res Clin Pract > Epub ahead of print |
Abstract
Background
Methods
Results
Conclusion
Notes
Funding
This study was supported by the Institute of Clinical Medicine Research of The Catholic University of Korea, Bucheon St. Mary’s Hospital Research Fund (BCMC20BD04).
Acknowledgments
Figure 1.
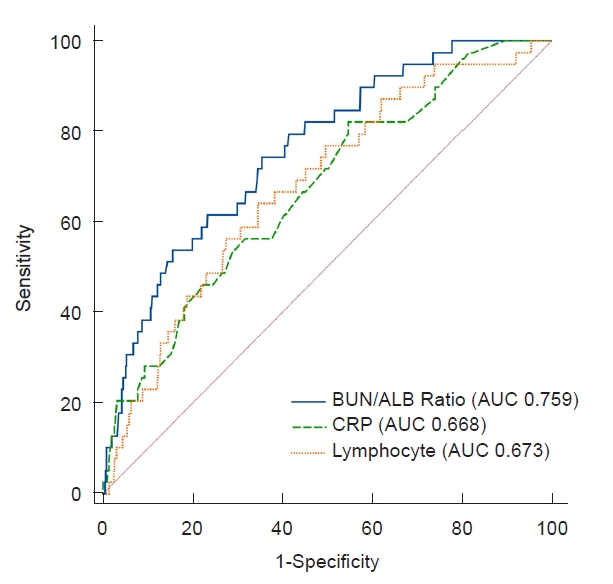
Table 1.
Table 2.
| Characteristic | Bacteremic group (n = 40) | Non-bacteremic group (n = 2,101) | p-value |
|---|---|---|---|
| Urine WBC (/HPF) | 0.51 | ||
| ≥50 | 32 (80.0) | 1,438 (68.4) | |
| 5–49 | 7 (17.5) | 525 (25.0) | |
| <5 | 1 (2.5) | 138 (6.6) | |
| Pathogen, urine | 0.53 | ||
| Escherichia coli | 39 (97.5) | 1,843 (87.7) | |
| Klebsiella spp. | 0 (0) | 98 (4.7) | |
| Enterococcus spp. | 0 (0) | 69 (3.3) | |
| Enterobacter spp. | 1 (2.5) | 52 (2.5) | |
| Others | 0 (0) | 39 (1.9) | |
| Resistance | 0.95 | ||
| ESBL | 6 (15.0) | 326 (15.5) | |
| Non-ESBL | 33 (82.5) | 1,667 (79.3) | |
| Unknown | 1 (2.5) | 108 (5.1) | |
| Serum, initial | |||
| WBC (/μL) | 14,175.8 ± 6,512.5 | 15,360.8 ± 5,880.1 | 0.26 |
| Neutrophil | 7,997.5 ± 4,842.4 | 7,540.5 ± 4,161.7 | 0.56 |
| Lymphocyte | 4,480.2 ± 2,001.3 | 5,855.2 ± 2,425.8 | <0.001 |
| Hemoglobin (g/dL) | 10.61 ± 1.24 | 10.94 ± 1.09 | 0.10 |
| Platelet (K/μL) | 388.7 ± 141.24 | 400.71 ± 115.25 | 0.60 |
| NLR | 1.90 ± 1.09 | 1.47 ± 1.02 | 0.02 |
| PLR | 101.49 ± 54.46 | 81.49 ± 60.15 | 0.03 |
| ESRa | 2.2 ± 1.55 | 1.48 ± 1.51 | 0.01 |
| CRPa | 21.32 ± 30.51 | 9.96 ± 11.35 | 0.02 |
| BUN (mg/dL) | 11.06 ± 3.46 | 8.74 ± 3.37 | <0.001 |
| Creatinine (mg/dL) | 0.26 ± 0.07 | 0.25 ± 0.07 | 0.46 |
| Albumin (g/dL) | 3.81 ± 0.28 | 4.11 ± 0.36 | <0.001 |
| BUN/albumin (mg/g) | 2.97 ± 0.86 | 2.14 ± 0.85 | <0.001 |
| AST (IU/L) | 30.50 (24.0–36.5) | 32.0 (26.0–41.0) | 0.17 |
| ALT (IU/L) | 22.0 (17.75–29.25) | 23.0 (17.0–32.5) | 0.78 |
| Abnormal kidney USG | 24/40 (60.0) | 882/2,084 (42.3) | 0.04 |
| Abnormal DMSA scan | 20/30 (66.7) | 786/1,287 (61.1) | 0.58 |
| VUR on VCUG | 4/21 (19.1) | 182/847 (21.5) | >0.99 |
Data are expressed as number (%), mean ± standard deviation, or median (interquartile range).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C-reactive protein; DMSA, 99m-Tc dimercaptosuccinic acid; ESBL, extended-spectrum β-lactamase; ESR, erythrocyte sedimentation rate; HPF, high power field; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; USG, ultrasonography; VCUG, voiding cystourethrography; VUR, vesicoureteral reflux; WBC, white blood cell count.
Table 3.
| Variable | Unadjusted OR (95% CI) | p-value | Adjusted ORa (95% CI) | p-value |
|---|---|---|---|---|
| Younger age | 1.33 (1.13–1.59) | <0.001 | 1.31 (1.12–1.70) | <0.001 |
| Serum, initial | ||||
| Lymphocyte | 1.00 (1.00–1.00) | <0.001 | 1.00 (1.00–1.00) | 0.005 |
| NRL | 1.34 (1.07–1.61) | 0.01 | ||
| PLR | 1.00 (1.00–1.01) | 0.05 | ||
| ESR | 1.30 (1.08–1.56) | 0.008 | ||
| CRP | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.01–1.05) | 0.005 |
| Hemoglobin | 0.75 (0.57–1.01) | 0.06 | ||
| Albumin | 0.08 (0.03–0.21) | <0.001 | 0.22 (0.07–0.71) | 0.01 |
| BUN | 1.11 (1.05–1.20) | 0.001 | 1.07 (1.00–1.18) | 0.04 |
| BUN/albumin ratio | 1.94(1.40–2.74) | <0.001 | ||
| Abnormal kidney USG | 2.02 (1.09–3.87) | 0.03 |
Table 4.
| Variable | Unadjusted OR (95% CI) | p-value | Adjusted ORa (95% CI) | p-value |
|---|---|---|---|---|
| Younger age | 1.18(0.95–1.46) | 0.13 | ||
| Serum, initial | ||||
| Lymphocyte | 1.00 (1.00–1.00) | <0.001 | 1.00 (0.99–1.00) | 0.001 |
| NRL | 1.41(1.12–1.73) | 0.006 | ||
| PLR | 1.00 (1.00–1.01) | 0.08 | ||
| ESR | 1.40 (1.15–1.70) | 0.001 | ||
| CRP | 1.04 (1.02–1.06) | <0.001 | 1.03 (1.01–1.05) | 0.003 |
| Hemoglobin | 1.00 (1.00–1.00) | 0.19 | ||
| Albumin | 0.09 (0.03–0.23) | <0.001 | 0.10 (0.03–0.33) | <0.001 |
| BUN | 1.15 (1.06–1.28) | 0.001 | 1.08 (1.01–1.22) | 0.04 |
| BUN/albumin ratio | 2.28 (1.55–3.33) | <0.001 | ||
| Abnormal kidney USG | 1.86 (1.00–3.56) | 0.05 |
References
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,194 View
- 25 Download
- ORCID iDs
-
Hyesun Hyun

https://orcid.org/0000-0001-8525-1471Yeon hee Lee

https://orcid.org/0000-0002-5816-6375Na Yoon Kang

https://orcid.org/0000-0002-5866-1261Jin-Soon Suh

https://orcid.org/0000-0002-6566-6618 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















