Drug-induced acute pancreatitis (DIAP) is rare, accounting for 0.1%â2% of acute pancreatitis, and is a diagnostic challenge to clinicians. Trimethoprim/sulfamethoxazole (TMP/SMX) is classified as a drug with the highest level of evidence [
1â
3]. Hypersensitivity is suggested as a possible mechanism and can be confirmed by the
in vitro proliferation of T cells to the drug in the lymphocyte transformation test (LTT).
Kidney transplant recipients (KTRs) receive TMP/SMX in the early days of transplantation and after antirejection treatment for
Pneumocystis jiroveci pneumonia (PJP) prophylaxis [
4]. However, no case of TMP/SMX-associated acute pancreatitis in a KTR has been reported yet. Here, we describe a case of TMP/SMX-induced pancreatitis confirmed by rechallenging and LTT in a KTR. This report was approved by the Institutional Review Board of Seoul St. Maryâs Hospital (No. KC23ZASI0083) and informed consent from the patient discussed in the report was obtained.
A 64-year-old man was admitted for preemptive living donor kidney transplantation (KT) preparation. He was treated with plasmapheresis plus intravenous immunoglobulin for desensitization due to ABO and human leukocyte antigen incompatibility. Seven days prior to KT, TMP/SMX at 80/400 mg twice daily was started with immunosuppressants (tacrolimus, mycophenolate mofetil, and prednisolone) for PJP prophylaxis. On the day of the planned KT, he complained of severe epigastric pain, requiring prompt evaluation and postponement of KT (
Fig. 1).
On physical examination, tenderness was found in the epigastrium with muscle guarding. The laboratory work-up showed a white blood cell count of 17,880/mm3, a C-reactive protein level of 0.08 mg/dL (reference, 0â0.5 mg/dL), and amylase and lipase levels of 1,742.0 U/L and 2,139.4 U/L, respectively (reference: amylase, 28â100 U/L; lipase, 13.0â60.0 U/L). Liver function tests were within normal limits. Serum triglyceride and immunoglobulin 4 (IgG4) levels were 45 mg/dL and 16.12 mg/dL (reference of IgG4, 3.9â86.4 mg/dL), respectively. Abdominal computed tomography showed peripancreatic fat infiltration with minimal parenchymal swelling. Therefore, the diagnosis of acute pancreatitis was made. Because there was no obvious cause of acute pancreatitis, DIAP was highly suspected. Then, possible etiologic drugs, such as TMP/SMX (class Ia), prednisolone (class II), and tacrolimus (class III) were withheld. After discontinuing the drugs, the patientâs abdominal pain subsided, along with a decrease in serum amylase and lipase levels.
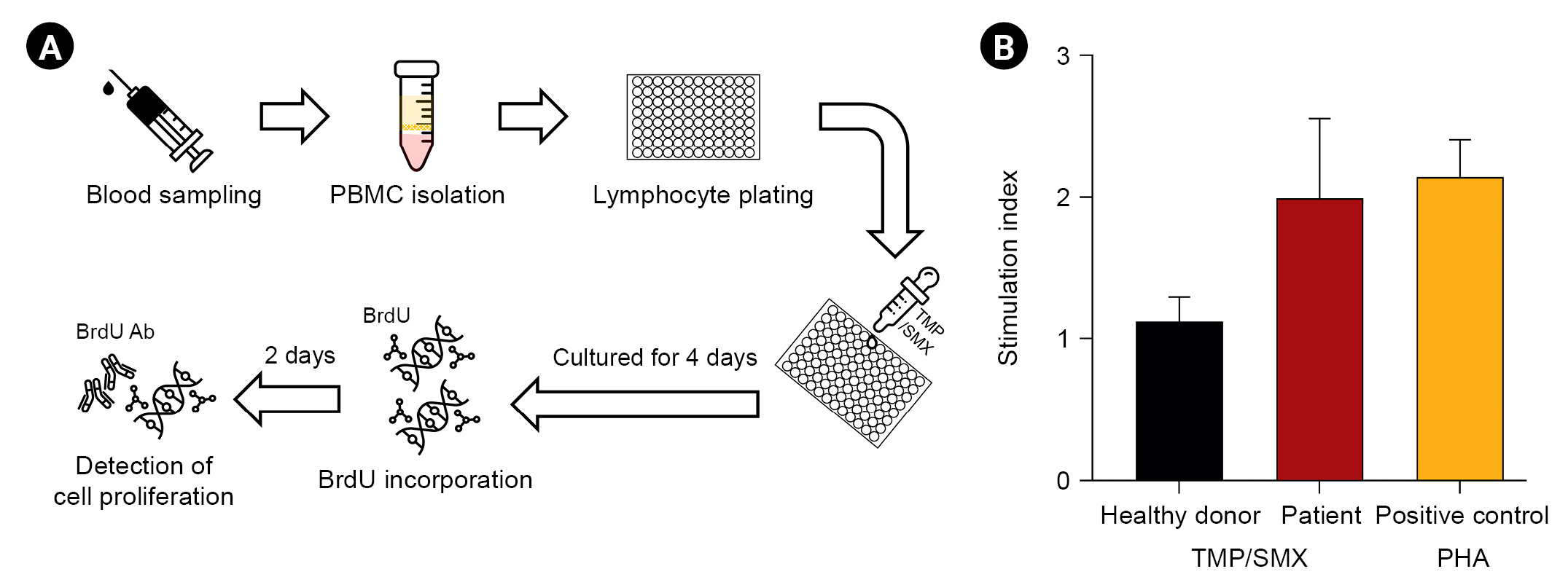
To confirm TMP/SMX as the culprit drug, we performed
in vitro tests using peripheral blood sampled from the patient and one healthy donor as the negative control (
Fig. 2A). Skin test was unavailable due to taking systemic steroid. Lymphocyte proliferation (stimulation index, 1.91 Âą 0.44) was enhanced in the concomitant incubation of TMP (100 Îŧg/mL) and SMX (500 Îŧg/mL) with peripheral blood mononuclear cells (PBMCs) from the patient (
Fig. 2B).
The diagnosis of TMP/SMX-associated acute pancreatitis was made for the patient. Therefore, he underwent KT without PJP prophylaxis after pancreatitis resolved. However, he developed PJP 2 months after transplant, and intravenous TMP/SMX was restarted. Five days after reexposure, serum amylase and lipase levels increased.
Acute pancreatitis is a rare but life-threatening complication in patients with transplanted kidneys if not properly managed. Even though drugs are rare causes of acute pancreatitis, a recent systematic review reported that more than 150 drugs are known to cause DIAP. TMP/SMX is classified as class Ia, with the highest level of evidence, but the evidence was mostly based on cause-and-effect relationships and repeated episodes of adverse events. Our case confirmed TMP/SMX as the culprit drug of acute pancreatitis episodes in a KTR through both positive re-challenge with the drug and lymphocyte stimulation.
The possible mechanisms of DIAP include hypersensitivity reactions, cellular toxicity of the drug or metabolites, and spasm of the sphincter of Oddi. Among them, the immunological basis of hypersensitivity reactions starts with the development of drug-specific memory T cells from naïve T cells after recognition of the drug peptide by major histocompatibility molecules. Then, when antigen-presenting cells present the specific drug peptide to memory T cells, the drug-specific T cells are activated and expanded [
5].
The LTT is an
in vitro test for T cell-mediated drug hypersensitivity, measuring the proliferation of drug-specific memory T cells following the coincubation of patient PBMCs with the offending drug [
6]. Although the drug rechallenging test is the gold standard for evaluating drug hypersensitivity, it is time-consuming and can carry risks for the patient. The above patient was diagnosed with TMP/SMX-induced pancreatitis by reexposure and positive LTT results. This indicates that TMP/SMX-induced DIAP could be associated with T cell-mediated hypersensitivity reactions, and LTT is helpful in confirming DIAP when the diagnosis is uncertain.
Our case uniquely reports TMP/SMX as a drug eliciting acute pancreatitis in a KTR. Moreover, the evidence was based on relapse after reexposure and positive LTT results. The results suggest that TMP/SMX should be considered a causative drug when acute pancreatitis occurs in KTRs, and LTT is helpful in diagnosing DIAP.
Acknowledgments
The authors thank Mr. So Young Park for her help with lymphocyte transformation test data.
Figure 1.
Clinical course of acute pancreatitis in the presence of TMP/SMX exposure.
ABOi, ABO incompatible; CP, clindamycin primaquine; FK, tacrolimus; HLAi, HLA incompatible; KT, kidney transplantation; MMF, mycophenolate mofetil; PJP, Pneumocystis jiroveci pneumonia; TMP/SMX, trimethoprim/sulfamethoxazole.

Figure 2.
Lymphocyte transformation test. (A) Steps of lymphocyte transformation test. (B) Proliferation of lymphocytes in response to trimethoprim/sulfamethoxazole (TMP/SMX; 100/500 Îŧg/mL) 6 days after drug stimulation measured by the bromodeoxyuridine (BrdU) incorporation assay. The stimulation index is the ratio of radioactivity counts in peripheral blood mononuclear cells (PBMCs) cultured with and without TMP/SMX. Phytohemagglutinin (PHA) is a potent T cell activator used as the positive control in the lymphocyte transformation test.
References
1. Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review.
Clin Gastroenterol Hepatol 2007;5:648â644.


2. Simons-Linares CR, Elkhouly MA, Salazar MJ. Drug-induced acute pancreatitis in adults: an update.
Pancreas 2019;48:1263â1273.


3. Jung M, Kim J, Lee JY, Kim M, Kim SH, Ahn K. Trimethoprim-sulfamethoxazole induces acute pancreatitis associated with drug-specific cytotoxic T lymphocytes.
J Allergy Clin Immunol Pract 2019;7:336â338.


4. Lee H, Han A, Choi C, et al. Proposal of a selective prophylaxis strategy based on risk factors to prevent early and late
Pneumocystis jirovecii pneumonia after renal transplantation.
J Korean Soc Transplant 2018;32:92â103.


5. Sachs B, Fatangare A, Sickmann A, Glässner A. Lymphocyte transformation test: history and current approaches.
J Immunol Methods 2021;493:113036.


6. Mayorga C, Celik G, Rouzaire P, et al. In vitro tests for drug hypersensitivity reactions: an ENDA/EAACI Drug Allergy Interest Group position paper.
Allergy 2016;71:1103â1134.














 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















