Pan-Nox inhibitor treatment improves renal function in aging murine diabetic kidneys
Article information
Abstract
Background
Aging is a risk factor for development of chronic kidney disease and diabetes mellitus with commonly shared features of chronic inflammation and increased oxidative stress. Here, we investigated the effect of pan-Nox-inhibitor, APX-115, on renal function in aging diabetic mice.
Methods
Diabetes was induced by intraperitoneal injection of streptozotocin at 50 mg/kg/day for 5 days in 52-week-old C57BL/6J mice. APX-115 was administered by oral gavage at a dose of 60 mg/kg/day for 12 weeks in nondiabetic and diabetic aging mice.
Results
APX-115 significantly improved insulin resistance in diabetic aging mice. Urinary level of 8-isoprostane was significantly increased in diabetic aging mice than nondiabetic aging mice, and APX-115 treatment reduced 8-isoprostane level. Urinary albumin and nephrin excretion were significantly higher in diabetic aging mice than nondiabetic aging mice. Although APX-115 did not significantly decrease albuminuria, APX-115 markedly improved mesangial expansion, macrophage infiltration, and expression of fibrosis molecules such as transforming growth factor beta 1 and plasminogen activator inhibitor 1. Interestingly, the expression of all Nox isoforms including Nox1, Nox2, and Nox4 was significantly increased in diabetic aging kidneys, and APX-115 treatment decreased Nox1, Nox2, and Nox4 protein expression in the kidney. Furthermore, Klotho expression was significantly decreased in diabetic aging kidneys, and APX-115 restored Klotho level.
Conclusion
Our results provide evidence that pan-Nox inhibition may improve systemic insulin resistance and decrease oxidative stress, inflammation, and fibrosis in aging diabetic status and may have potential protective effects on aging diabetic kidney.
Introduction
Aging and diabetic kidney disease share many similar pathogenic mechanisms such as oxidative stress, inflammation, cellular senescence, and mitochondrial dysfunction [1,2]. Increasing evidence indicates that the main mediator for cellular senescence is oxidative stress derived from imbalance between production and scavenging of reactive oxygen species (ROS) [3,4]. Increased advanced oxidation protein products bind to receptor for advanced glycation end to activate downstream signaling pathways such as nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase (Nox) [5].
Diabetes is the leading cause of end stage renal disease. Although many factors such as hypertension, hyperglycemia, and genetic factors contribute to the development of diabetic kidney disease, oxidative stress is a basic pathogenic mechanism for initiation and progression of diabetic kidney disease [6]. There is growing evidence suggesting that renal oxidative stress is related to the activation of various Nox isoforms [6–9]. Previous reports have shown that activation of Nox causes mesangial cell hypertrophy and extracellular matrix expansion and podocyte apoptosis [6]. Furthermore, activation of Nox in renal tubular cells contributes to the development of tubulointerstitial inflammation and fibrosis [10–12].
There are seven Nox isoforms (Nox1–5, dual oxidase [Duox] 1, Duox2), among which Nox1, Nox2, Nox4, and Nox5 are widely present in renal tissue and cells [13]. Among these Nox isoforms, Nox4 play a critical role in diabetes-associated oxidative stress injury. Many studies have investigated Nox4 as a potential target in experimental model of diabetic nephropathy [14–17], and targeting Nox has been expected as a promising therapy in diabetic nephropathy [18,19]. We previously reported that the novel pan-Nox-inhibitor APX-115 improved renal function and histology in experimental animal model of type 1 and 2 diabetes [20,21]. However our previous studies observed the renoprotective effects of pan-Nox-inhibitor in young diabetic mice, and we recently reported that three Nox isoforms, namely Nox1, Nox2, and Nox4, was significantly increased during aging process especially diabetic condition [22]. Since aging kidney may be more difficult to prevent the progression of renal disease, we performed this experiment in an experimental aging diabetic murine model to investigate whether targeting of these oxidases might be a new promising therapy in aging diabetic kidney disease.
Methods
All experiments were conducted in accordance with National Institutes of Health guidelines and with the approval of the Korea University Institutional Animal Care and Use Committee (No. KOREA-2020-0036).
Animal studies
Animal experiments were performed as described previously [20,21]. C57BL6 mice were purchased from Central Lab Animal Inc. Diabetes was induced by intraperitoneal (i.p.) injection of streptozotocin (STZ) at 50 mg/kg/day for 5 days in 52-week-old C57BL/6J mice, and age-matched control mice were injected with an equivalent volume of sodium citrate buffer (100-mmol/L sodium citrate, 100-mmol/L citric acid, pH 4.5). The mice had free access to tap water and food (standard chow; Cargill Agri Purina Korea Inc.) and were caged individually under controlled temperature (23 ± 2 °C) and humidity (55% ± 5%). APX-115 was kindly supplied from AptaBio Therapeutics Inc., and the chemical characteristics of APX-115 were previously described [23].
To investigate the effect of APX-115, the mice were divided into four groups at the age of 52 weeks: 1) nondiabetic aging control (n = 9), 2) nondiabetic aging treated with APX-115 control (n = 7), 3) diabetic aging treated with vehicle (n = 14), and 4) diabetic aging treated with APX-115 (n = 13). APX-115 was administered by oral gavage at a dose of 60 mg/kg/day for 12 weeks. Body weight, food and water intake, fasting glucose concentration, urine volume, and glycated hemoglobin (HbA1c) level were measured every month. Plasma glucose concentration was determined by glucose oxidase method, and HbA1c concentration was measured using the IN2IT system (Bio-Rad Laboratories). Plasma insulin level was determined by an enzyme-linked immunosorbent assay (ELISA) kit (Linco Research). Serum creatinine concentration was measured using modified Jaffe method. The homeostasis model assessment index of insulin resistance (HOMA-IR) was calculated by the formula of fasting glucose concentration (mmol/L) × fasting insulin concentration (mU/L) / 22.5. Plasma lipoprotein profiles were measured by high-performance liquid chromatography system. Plasma triglyceride and cholesterol analyses were done using a GPO-Trinder kit (Sigma). For the glucose tolerance test, we performed oral gavage of 3 g dextrose/kg in mice after a 5-hour fast, collected blood samples through the tail vein, and glucose concentration was measured at 0, 30, 60, 90, and 120 minutes. Insulin tolerance testing (ITT) was performed after an 8-hour fast, and blood was collected through the tail vein. Mice received 0.75 U/kg regular insulin by i.p. injection, and glucose concentration was subsequently measured at 0, 30, 60, 90, and 120 minutes. Individual mice were separated in a metabolic cage where urine was collected and measured the amount of urinary albumin excretion using a competitive ELISA kit (ALPCO) at every month. Urinary nephrin concentration was measured using an ELISA kit (Exocell). Serum cystatin C concentration were measured by an ELISA kit (ALPCO). Plasma and urinary concentrations of 8-isoprostane were determined by an ELISA kit (Cayman Chemical). At the end of the study period, systolic blood pressure (SBP) was determined by tail-cuff plethysmography (LE 5001-Pressure Meter; Letica S.A.). All ELISA analyses were performed in duplicate, and the results were averaged. Mice were sacrificed under anesthesia through i.p. injection of tribromoethanol (Avertin, 50 mg/kg; Sigma-Aldrich), and kidney tissues were weighed and subsequently snap frozen in liquid nitrogen.
Histological and immunohistochemical analyses
Histologic and immunohistochemical experiments were performed as described previously [21]. Kidney tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Renal tissues were cut into 4-μm-thick slices and stained with periodic acid-Schiff. For immunohistochemical staining, sections were microwaved for 10 to 20 minutes to retrieve antigens for transforming growth factor beta 1 (TGF-β1) and F4/80 staining. For plasminogen activator inhibitor 1 (PAI-1) staining, slides were transferred to Biogenex Retrievit buffer (pH 8.0; InnoGenex) and microwaved for 10 to 20 minutes for PAI-1. To block endogenous peroxidase activity for 20 minutes, tissue sections were treated with methanol containing 3.0% H2O2. Samples were then incubated for 15 minutes at room temperature with 10% powerblock for PAI-1, for 30 minutes with 20% normal sheep serum for TGF-β1, or 60 minutes in 3% BSA/3% normal goat serum for F4/80. Slides were then incubated overnight with rabbit polyclonal anti-TGF-β1 antibodies (1:200; Santa Cruz Biotechnology Inc.) at 4 °C, rabbit polyclonal anti-PAI-1 antibodies (1:60; American Diagnostica) or mouse monoclonal anti-F4/80 antibodies (1:100; Serotec Inc.). After overnight incubation, slides were then incubated with secondary antibodies for 30 minutes, and immunoreactive areas were detected by incubation with a mixture of 0.05% 3,3-diaminobenzidine containing 0.01% H2O2 at room temperature, followed by counterstaining with Mayer’s hematoxylin. Negative control slides were stained under same conditions but with a buffer solution without primary antibody. Scoring of glomerular mesangial expansion was determined by semiquantitative method, and the percentage of mesangial matrix occupying each glomerulus was scored from 0 to 4 as follows: 0, 0%; 1, <25%; 2, 25% to 50%; 3, 50% to 75%; and 4, >75% as described previously [24]. For scoring of the immunohistochemical staining for TGF-β1 and PAI-1, glomerular fields containing 50–60 glomeruli were scored semiquantitatively under a high-power field, and an average score was measured as described previously [24]. Infiltrating macrophages in the interstitium were counted and scored as the number of macrophages per high-power field. All histologic examinations were conducted in a blinded manner by a trained pathologist.
Protein extraction and Western blot analysis
Methods of Western blotting is described previously [21]. For Western blotting, 40 μg of protein was electrophoresed on an 8% to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis minigel. Proteins were transferred onto a polyvinylidene difluoride membrane, and the membrane was hybridized in blocking buffer overnight at 4 °C with rabbit polyclonal anti-Nox1 antibody (1:1,000; Abcam Plc), rabbit polyclonal anti-Nox2 antibody (1:1,000; Bioworld Technology), rabbit polyclonal anti-Nox4 antibody (1:1,000; Bioworld Technology), rabbit polyclonal anti-PAI-1 antibody (1:1,000; Santa Cruz Biotechnology), mouse monoclonal anti-monocyte chemoattractant protein (MCP)-1 antibody (1:1000; Lsbio), rabbit polyclonal anti-type IV collagen antibody (1:1,000; Abcam Plc), rabbit polyclonal anti-TGF-β1 antibody (1:1,000; Abbkine), goat polyclonal anti-Klotho antibody (1:1,000; R&D Systems Inc.), rabbit polyclonal anti-phospho FoxO1 antibody (1:1,000; Lsbio), rabbit polyclonal anti-total-FoxO1 antibody (1:1,000; Cell Signaling Technology), rabbit polyclonal anti-NRF2 antibody (1:1,000; Cell Signaling Technology), or mouse monoclonal anti-β actin antibody (1:5,000; Sigma-Aldrich). The membrane was subsequently incubated with horseradish peroxidase-conjugated secondary antibody (1:1,000 dilution) for 60 minutes at room temperature. The detection of specific signals was performed using an enhanced chemiluminescence method (Amersham).
Statistical analysis
Nonparametric analysis was used due to the relatively small number of samples present. Results are expressed as mean ± standard error of mean. Comparisons were performed using Wilcoxon rank sum tests and Bonferroni correction. The Kruskal-Wallis test was used for comparison of more than two groups, followed by a Mann-Whitney U test. A p-value of < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS for Windows, version 20.0 (IBM Corp.).
Results
Physical and biochemical parameters in experimental animals
Table 1 shows the physical and biochemical results of our experimental animal model. The STZ-induced diabetic aging groups showed significantly lower body weight and higher food intake, water intake, urine volume, blood glucose concentration, and HbA1c level compared to the nondiabetic aging controls as expected. These parameters were not different between the APX-115-treated and vehicle groups except for HbA1c level, which was significantly decreased in the APX-115-treated diabetic aging group. Although serum creatinine levels did not differ among groups, serum cystatin C concentrations were significantly decreased in the APX-115-treated diabetic aging group (Table 1). SBP and serum creatinine concentration did not show any significant difference among the groups.
Effects of APX-115 on urinary albumin, nephrin excretion, and oxidative stress in experimental animals
Urinary excretion of albumin was significantly higher in the diabetic aging group than in nondiabetic aging controls during the entire study period (Fig. 1A). Urinary nephrin showed similar changes and was significantly higher in that diabetic aging group than in nondiabetic aging controls (Fig. 1B). Although plasma level of 8-isoprostane did not show a significant difference, urinary 8-isoprostane was significantly higher in the diabetic aging group (Fig. 1C, D). APX-115 treatment tended to decrease urinary excretion of albumin and nephrin, but the results were not statistically significant (Fig. 1A, B). In the nondiabetic aging group, APX-115 reduced plasma 8-isoprostane concentration, which was not decreased in the diabetic aging group (Fig. 1C); however, urinary excretion of 8-isoprostane was significantly decreased in the diabetic group by APX-115 treatment (Fig. 1D).

Urinary excretion of albumin and nephrin and oxidative stress change in experimental animals.
(A) Urinary albumin concentration, (B) urinary nephrin concentration, (C) plasma 8-isoprostane concentration, and (D) urinary 8-isoprostane concentration. Data are shown as the mean ± standard error of mean. HPF, high power field.
*p < 0.05 vs. control, **p < 0.01, ***p < 0.001 vs. control or APX-115, #p < 0.05 vs. streptozotocin (STZ).
Effect of APX-115 on insulin resistance in experimental animals
We examined whether APX-115 improved metabolic profiles and insulin resistance. Whereas plasma lipid parameters were not different among the groups (Fig. 2A), APX-115 improved glucose intolerance in the nondiabetic aging group (Fig. 2B). Insulin resistance determined by the ITT showed that APX-115 significantly decreased glucose concentration after insulin injection in both nondiabetic aging and diabetic aging groups (Fig. 2C). This result was consistent with HOMA-IR level, another index of insulin resistance (Fig. 2D).
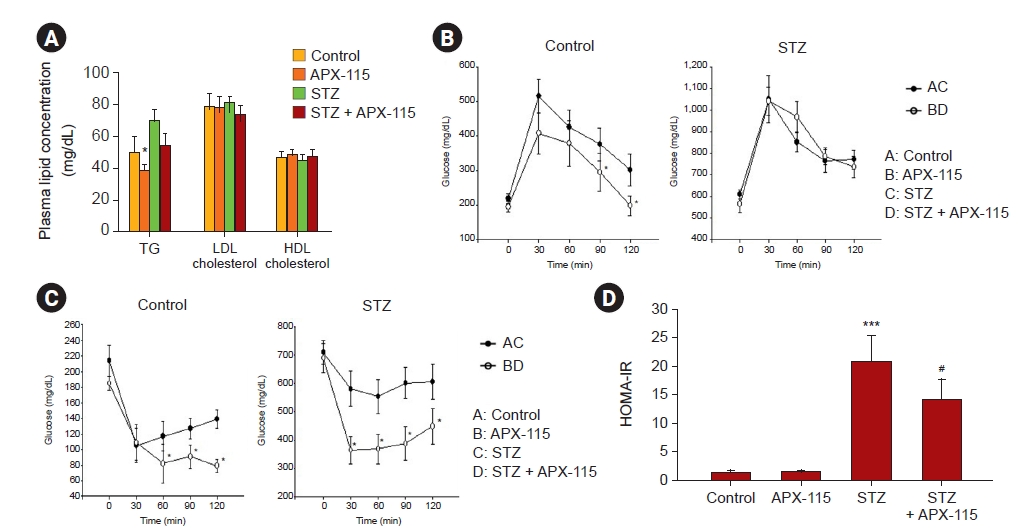
Plasma lipid levels, glucose tolerance test, insulin tolerance test, and HOMA-IR in experimental animals.
(A) Plasma lipid concentration, (B) glucose tolerance test, (C) insulin tolerance test, and (D) HOMA-IR. Data are shown as the mean ± standard error of mean.
HOMA-IR, homeostasis model assessment index of insulin resistance; STZ, streptozotocin.
*p < 0.05 vs. control, ***p < 0.001 vs. control or APX-115, #p < 0.05 vs. STZ.
Effects of APX-115 on inflammatory and fibrotic processes in experimental animals
Fig. 3 shows the representative changes in renal histology in experimental animals. Mesangial expansion was significantly increased in the diabetic kidney and was markedly attenuated with APX-115 treatment (Fig. 3 and 4A). As shown in Fig. 4B, macrophage infiltration, as measured by F4/80 staining, was remarkably increased in the diabetic kidney, and APX-115 treatment significantly reduced macrophage infiltration (Fig. 3 and 4B). Immune reactivity with profibrotic molecules such as PAI-1 and TGF-β1 was markedly increased in the diabetic kidney and was significantly improved with APX-115 treatment (Fig. 3 and 4C, D).
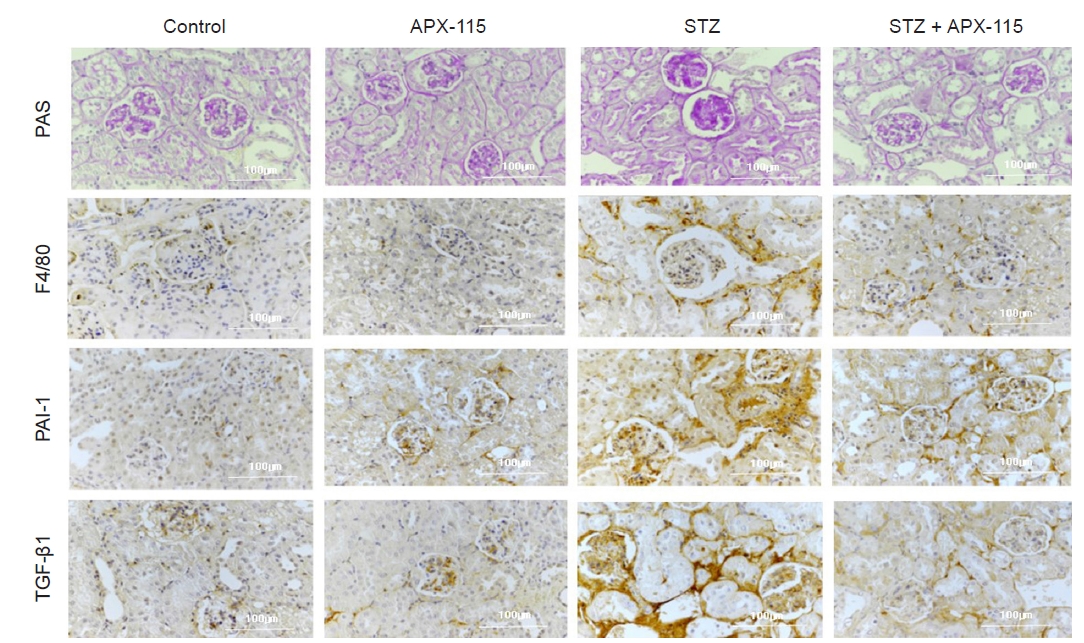
Representative renal immunohistochemistry findings in experimental animals.
F4/80, F4/80 stain; PAI-1, plasminogen activator inhibitor-1 stain; PAS, periodic acid-Schiff stain; STZ, streptozotocin; TGF-β1, transforming growth factor beta-1 stain. Original magnification, ×400.

Immunohistochemical (IHC) staining score.
(A) Mesangial expansion score, (B) scoring for F4/80-positive cells, (C) IHC staining score for plasminogen activator inhibitor 1 (PAI-1), and (D) IHC staining score for transforming growth factor beta 1 (TGF-β1). Data are shown as the mean ± standard error of mean.
***p < 0.001 vs. control or APX-115, #p < 0.05, ##p < 0.01, ###p < 0.001 vs. streptozotocin (STZ).
Effects of APX-115 on Nox isoforms and Klotho expression in experimental animals
We next performed Western blots for inflammatory and fibrotic molecules. Although TGF-β1 expression was not increased in diabetic kidneys, MCP-1 expression was significantly increased in the diabetic kidney and was suppressed by APX-115 treatment (Fig. 5A, B). As the renal effects of APX-115 were remarkable, we analyzed Nox expression in the kidney. Interestingly, all Nox isoforms, including Nox1, Nox2, and Nox4, were significantly increased in the diabetic kidney, and APX-115 significantly attenuated Nox1, Nox2, and Nox4 protein expression (Fig. 5C, D). We also found that PAI-1 and type IV collagen expression was increased in the diabetic kidney, and levels were decreased by APX-115 treatment (Fig. 6A, B). On the contrary, Klotho expression was significantly decreased in the diabetic kidney and restored by APX-115 treatment (Fig. 6C, D). Finally, we performed Klotho related oxidative stress markers such as FoxO1 and Nrf2 expression. FoxO1 phosphorylation was increased in diabetic kidney, whereas APX-115 suppressed FoxO1 phosphorylation. In contrast, Nrf2 expression was significantly decreased in diabetic kidney, and restored with APX-115 treatment (Fig. 6C, D).
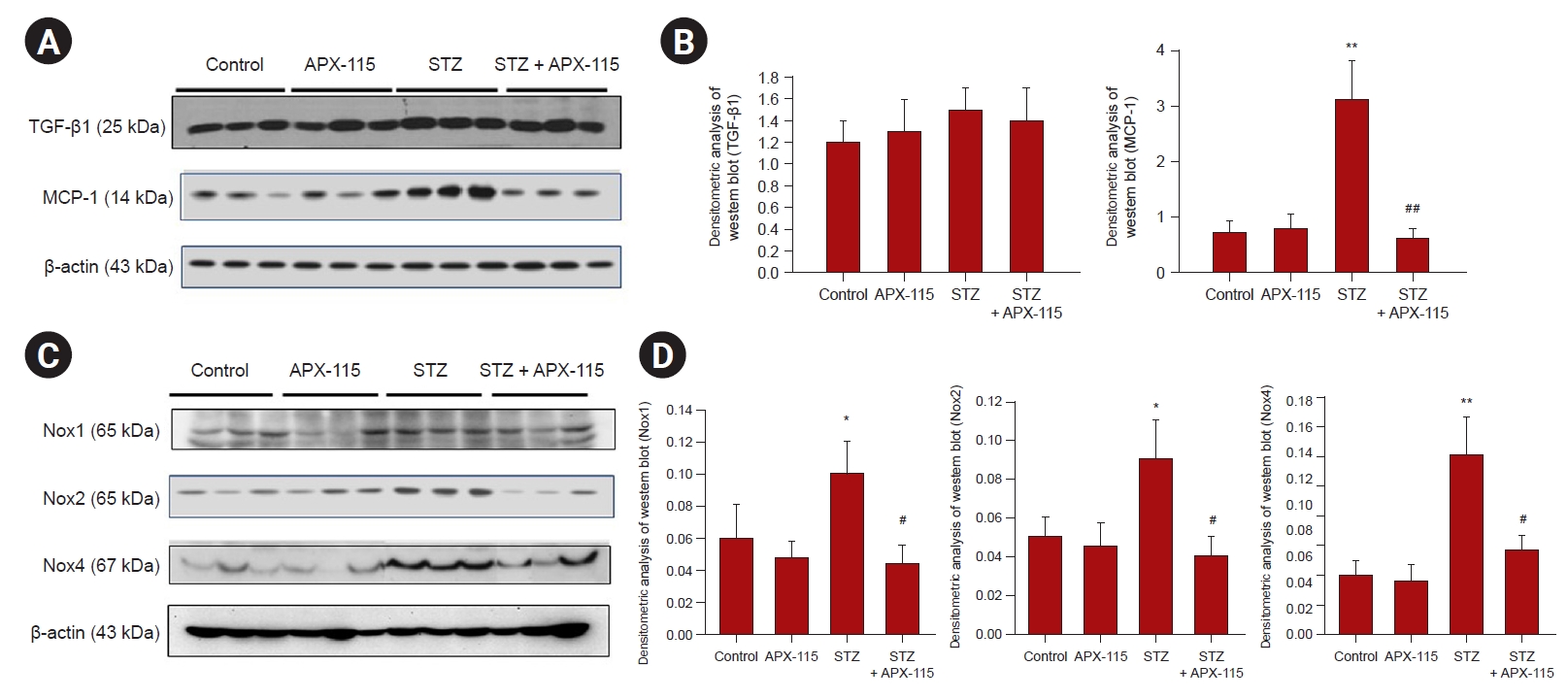
Representative immunoblots from renal cortical tissues in experimental animals.
(A) Representative immunoblots for transforming growth factor beta 1 (TGF-β1) and monocyte chemoattractant protein 1 (MCP-1); (B) densitometric analysis of immunoblots for TGF-β1 and MCP-1; (C) representative immunoblots for Nox1, Nox2, and Nox4; and (D) densitometric analysis of immunoblots for Nox1, Nox2, and Nox4. Data are shown as the mean ± standard error of mean.
Nox, nicotinamide adenine dinucleotide phosphate oxidase; STX, streptozotocin.
*p < 0.05, **p < 0.01 vs. control or APX-115, #p < 0.05, ##p < 0.01 vs. STZ.

Representative immunoblots from renal cortical tissues in experimental animals.
(A) Representative immunoblots for plasminogen activator inhibitor 1 (PAI-1) and type IV collagen (Col4); (B) densitometric analysis of immunoblots for PAI-1 and Col4; (C) representative immunoblot for Klotho, p-FoxO1, t-FoxO1 and Nrf2; and (D) densitometric analysis of immunoblot for Klotho, p-FoxO1/t-FoxO1 ratio, Nrf2. Data are shown as the mean ± standard error of mean.
*p < 0.05, ***p < 0.001 vs. control or APX-115, #p < 0.05, ###p < 0.001 vs. streptozotocin (STZ).
Discussion
Our study provides evidence that APX-115 exerts renoprotective effects in diabetic aging mice, significantly improving systemic insulin resistance and renal oxidative stress. In the kidney, APX-115 improved diabetes-related structural changes such as mesangial expansion, macrophage infiltration, and expression of fibrotic molecules. Furthermore, APX-115 attenuated Nox1, Nox2, and Nox4 expression and restored Klotho expression in aging diabetic kidneys.
Aging is associated with various functional and structural changes in the kidney that are aggravated by combined systemic disease such as hypertension and diabetes mellitus or other underlying kidney diseases. Because diabetes mellitus is rapidly increasing worldwide and diabetic kidney disease is the leading cause of end stage renal disease, we used a diabetic aging murine model in this study.
Over the past decade, accumulating evidence supports a role of Nox levels in renal inflammation and fibrosis. Of the seven Nox isoforms, Nox1, Nox2, Nox4, and Nox5 are expressed in the glomerular cells and tubulointerstitial cells. Among these isoforms, Nox5 is not detected in murine kidney tissues and was not examined in this study. We observed that Nox1, Nox2, and Nox4 are significantly upregulated in the diabetic kidney, and APX-115 induced significant reduction in the expression of all present Nox isoforms. Although Nox4 is most widely expressed in the kidney and has been the focus in diabetic kidney disease, recent studies suggest an important pathogenetic role of Nox1 in the progression of diabetic atherosclerosis [25,26]. Altogether, these results suggest that various Nox isoforms play different roles in the pathogenesis of diabetic vascular complications, and more generalized inhibition of ROS generation may be a promising therapeutic strategy to improve diabetic vasculopathy.
In the present study, we found that APX-115 treatment markedly improved insulin resistance including reduced HbA1c level and HOMA-IR index in diabetic mice. Improvement in insulin resistance was further confirmed by ITTs in diabetic mice. These results are in line with our previous reports [21]. We also observed that serum cystatin C concentration was significantly decreased by APX-115-treatment. However, we did not observe significant changes in SBP in experimental animals. We also found significantly higher urinary level of 8-isoprostane in the diabetic group, and APX-115 treatment markedly suppressed urinary 8-isoprostane level, which reflects the improvement in renal oxidative stress.
We observed that urinary excretion of albumin and nephrin was markedly increased in diabetic mice. Although APX-115 reduced albuminuria and urinary nephrin excretion, there was no statistical significance. Compelling evidence suggests that podocytes are the principal target glomerular cell in the aging process. Floege et al. [27] reported that podocytes are the main cells involved in age-related glomerulosclerosis, as urinary loss of podocytes is accompanied by decrease in podocyte number and density with aging [28,29]. Increased urinary excretion of nephrin in diabetic mice in this study may imply that podocyte aging is accelerated in the diabetic condition.
The most important finding of this study is that APX-115 treatment decreased mesangial expansion in renal tissues, accompanied by suppression of profibrotic and proinflammatory molecules. We observed that expression of Nox1, Nox2, and Nox4 was significantly increased in diabetic mice, associated with increase in collagen IV and PAI-1 expression and macrophage infiltration. APX-115 significantly attenuated Nox1, Nox2, and Nox4 protein expression to improve renal inflammation and fibrosis.
Another interesting finding in this study is that Klotho expression was markedly decreased in the diabetic kidney, and APX-115 treatment restored Klotho expression. There is accumulating evidence suggesting that Klotho expression is reduced during increased oxidative stress [30], and oxidative stress also reduces Klotho expression in renal tubular cells [31]. Taken together, these results imply that Klotho plays an important role in renal fibrosis and oxidative stress in the aging kidney. In addition, we also observed the increased FoxO1 phosphorylation and decreased Nrf2 expression in aging diabetic kidney, whereas APX-115 treatment restored these changes. Taken together, these results suggest that APX-115 treatment provides a potential anti-aging therapy.
Because Nox2 is widely expressed in monocytes/macrophages and plays an important role in protecting against bacterial infections, concern must be raised about the undesirable infectious side effects of Nox2 inhibition [32]. However, in our experimental model, APX-115 did not show susceptibility to infections or show any lethality. A limitation in this study is that age of the experimental mice at sacrifice was 64 weeks, representing middle age. Since we could not obtain mice at 24 months of age, we used mice at 52 weeks of age in this study.
In conclusion, our findings provide evidence that pan-Nox inhibition by APX-115 may have renoprotective potential in aging diabetic kidney disease. These findings suggest that APX-115 may be a useful new therapeutic agent in the treatment of aging and diabetes.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C0293), and by the Aging Project (No. 2017M3A9D8062955) funded by the Ministry of Science and ICT.
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: SYH, DRC
Data curation: SYH, JJC
Formal analysis: JJC, YSK, SYH, YJS, JYH
Funding acquisition: DRC
Investigation: JYG, JAY, JYH
Methodology: SGY, JYG, JAY
Writing–original draft: JHP
Writing–review & editing: JHP, DRC
Acknowledgements
We thank the AptaBio Therapeutics Inc. for kindly providing APX-115. We also declare that this provision had no impact on the research results in any way.

