| Kidney Res Clin Pract > Volume 42(6); 2023 > Article |
|
Abstract
Background
Methods
Results
Supplementary Materials
Notes
Funding
The current study received grants from the National Natural Science Foundation of China (grant number: 82072222), the Fundamental Research Funds for the Central Universities, China (grant number: 3332019127), the Science and Technology Fund of Tianjin Municipal Health Bureau (grant number: ZC20180), and the Science and Technology Fund of Tianjin Municipal Education Commission (grant number: 2021KJ216).
Data sharing statement
All the data supporting the findings of this study are included in the article.
AuthorsŌĆÖ contributions
Conceptualization: YZ, AM
Data curation: WW, YZ, AM, KS, XL
Formal analysis: KS
Funding acquisition: HJ
Investigation: WW, KS
Methodology: XL, YZ
Project administration: HJ, QL, SS, YZ
Software: WW, YZ, AM, QL
Supervision: HJ, SS, YZ
Validation: SS
WritingŌĆōoriginal draft: WW, YZ
WritingŌĆōreview & editing: HJ, SS
All authors read and approved the final manuscript.
Acknowledgments
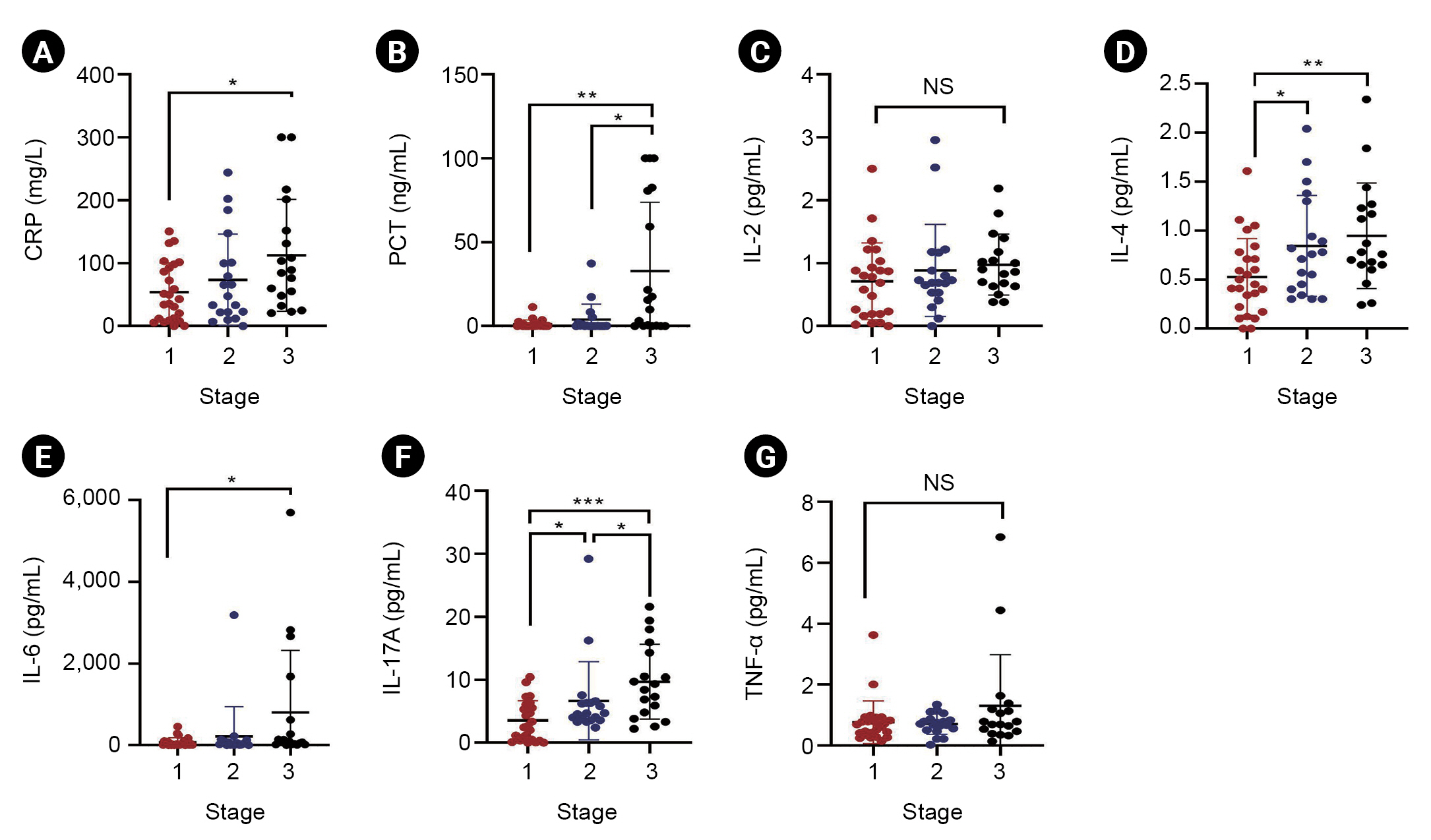
Figure┬Ā1.
Risk stratification of inflammatory cytokines in SAKI patients.
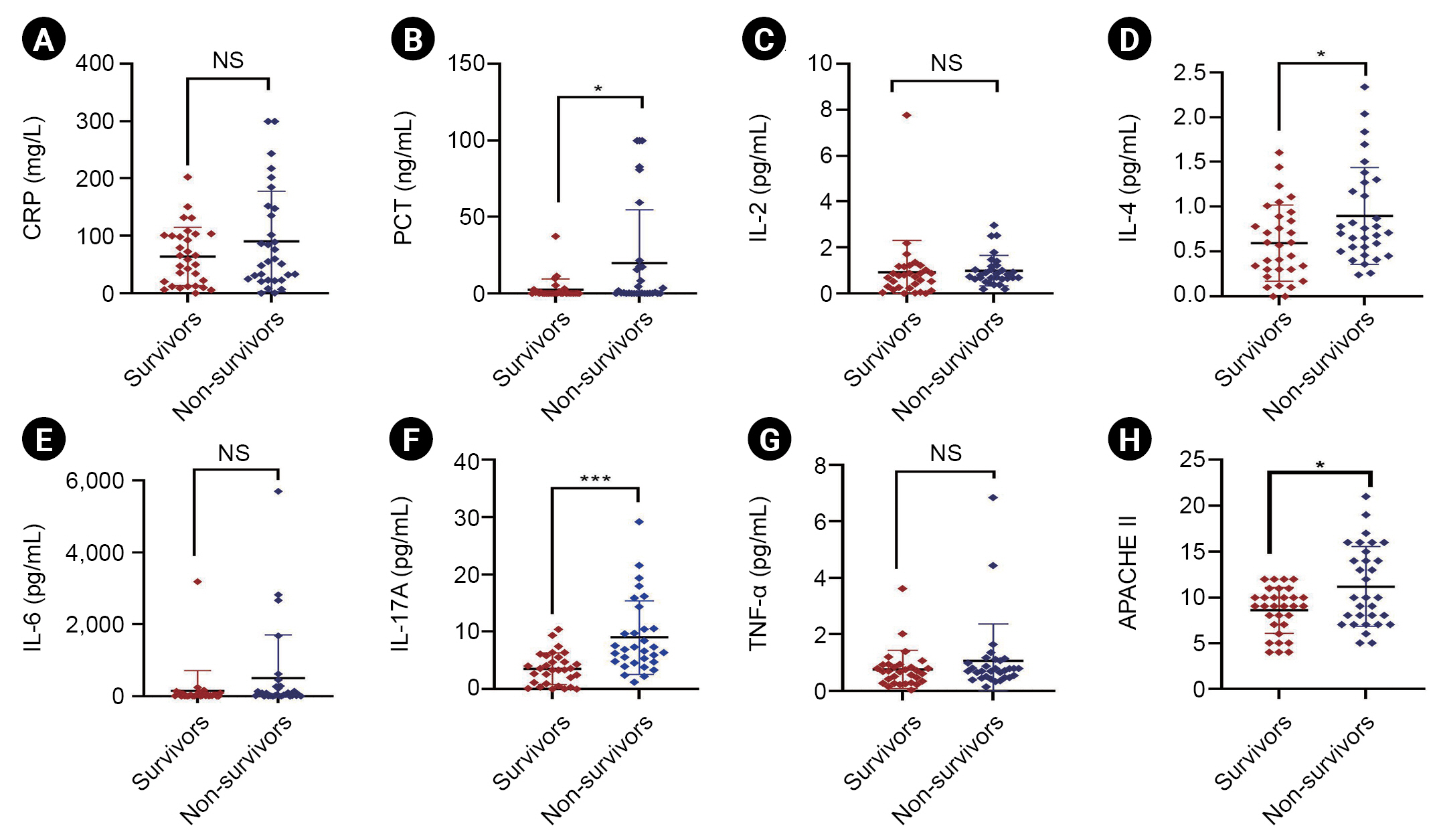
Figure┬Ā2.
Prognosis of inflammatory cytokines in SAKI patients.
Figure┬Ā3.
Role of IL-17A in the prognosis of SAKI patients.

Table┬Ā1.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,547 View
- 101 Download
- ORCID iDs
-
Heng Jin

https://orcid.org/0000-0001-9264-1187Wei Wei

https://orcid.org/0000-0001-7400-7502Yibo Zhao

https://orcid.org/0000-0002-7490-3865Ai Ma

https://orcid.org/0000-0002-5241-8295Keke Sun

https://orcid.org/0000-0001-5335-2825Xiaoxi Lin

https://orcid.org/0000-0002-4000-0131Qihui Liu

https://orcid.org/0000-0003-1263-0176Songtao Shou

https://orcid.org/0000-0002-9959-3963Yan Zhang

https://orcid.org/0000-0002-6610-5345 - Related articles
-
Nephrology consultation improves the clinical outcomes of patients with acute kidney injury



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















