Autophagy and regulation of aquaporins in the kidneys
Article information
Abstract
Aquaporins (AQPs) are water channel proteins that facilitate the transport of water molecules across cell membranes. To date, seven AQPs have been found to be expressed in mammal kidneys. The cellular localization and regulation of the transport properties of AQPs in the kidney have been widely investigated. Autophagy is known as a highly conserved lysosomal pathway, which degrades cytoplasmic components. Through basal autophagy, kidney cells maintain their functions and structure. As a part of the adaptive responses of the kidney, autophagy may be altered in response to stress conditions. Recent studies revealed that autophagic degradation of AQP2 in the kidney collecting ducts leads to impaired urine concentration in animal models with polyuria. Therefore, the modulation of autophagy could be a therapeutic approach to treat water balance disorders. However, as autophagy is either protective or deleterious, it is crucial to establish an optimal condition and therapeutic window where autophagy induction or inhibition could yield beneficial effects. Further studies are needed to understand both the regulation of autophagy and the interaction between AQPs and autophagy in the kidneys in renal diseases, including nephrogenic diabetes insipidus.
Introduction
Under physiological conditions, the proteins in the cells or body undergo constant turnover and changes in intracellular protein synthesis and degradation. The fine balance of the protein pool is maintained by several adaptive mechanisms, e.g., the activation of autophagy, endoplasmic reticulum-associated degradation, and an unfolded protein response [1,2]. Protein localization at the membrane is maintained by the interaction between the cytosolic domains of membrane proteins and cytosolic proteins. Membrane protein expression (e.g., transporters and channels) is usually highly dynamic due to protein trafficking between the membrane and cytoplasm and posttranslational modification. Autophagy, a highly conserved lysosomal-dependent degradation pathway of proteins, plays an important role in preserving cellular homeostasis. The importance of autophagy in kidney disease and homeostasis has been previously reviewed [3]. Emerging evidence suggests that autophagy may influence tubular transport in the kidney.
In this article, we aimed to provide an overview of the physiological and pathophysiological roles of autophagy in kidney diseases and, in particular, the current evidence on the pathophysiological roles of autophagy in tubular transport. The therapeutic potentials and challenges are also discussed when autophagy is targeted for the prevention and treatment of tubular dysfunction in kidney diseases.
Overview of the autophagic process
Autophagy is a highly conserved pathway of degrading organelles and proteins in eukaryotes. Autophagy is strictly regulated by autophagy-related genes (ATGs) and many other genes. In autophagy, cellular components, e.g., organelles or proteins, are wrapped in autophagosomes with a double-layer membrane structure, which later fuse with lysosomes for degradation [4]. Thereafter, the degraded products, such as carbohydrates and amino acids, are re-released into the cytoplasm to synthesize new amino acids, nucleotides, and organelles, and they can also provide cells with energy to maintain their basic survival [5,6]. Under basic conditions, only a low level of autophagy is involved in the degradation of damaged or aging organelles, misfolded proteins, and protein aggregates to preserve the normal survival and function of cells [7]. In contrast, when cells are damaged or stimulated by several factors, such as oxidative stress, hypoxia, DNA damage, chemicals, nutrient deprivation, and intracellular microorganisms, autophagy is activated. Autophagy in the body is, therefore, crucial for cell proliferation, differentiation, metabolism, and other physiological processes.
Autophagy was first demonstrated as a protective pathway against starvation conditions, while later evidence indicates that autophagy also contributes to the dysfunction of the cells and the damage to the organs. Three types of autophagy have been categorized, which are dependent on the delivery routes of substrates to the lysosomes, including macroautophagy (or autophagy), microautophagy, and chaperone-mediated autophagy (CMA) [3].
Macroautophagy or autophagy involves multiple steps including initiation, nucleation, expansion, fusion, and degradation [3]. The canonical activation route is induced by formation of a protein complex composed of serine/threonine protein kinases ULK1, ULK2, and other protein partners [3,8]. Activation of the ULK1 induces phosphorylation of the phosphoinositide 3-kinase complex and recruitment of intracellular membranous domains (e.g., endoplasmic reticulum [ER], plasma membrane) to form the omegasome [9]. Two ubiquitin (Ub)-like conjugation systems, the ATG12–ATG5–ATG16L system and the microtubule-associated protein 1 light chain 3 (LC3) system, control the expansion and completion of the autophagosome [10], supporting the maturation of the omegasome into the phagophore. The phagophore segregates the cytoplasmic cargoes and closes upon itself to form the autophagosome [9]. The sequestration of cytoplasmic components could be either selective or nonselective. Selectivity in autophagy could be induced by exploiting autophagy receptors, such as SQSTM1 (p62) and optineurin, to carry specific proteins/organelles to the phagophores [9]. Autophagosomes then merge with early and/or late endosomes and finally fuse with lysosomes, forming the autolysosome [11]. Autolysosomes are not perpetual. When cargo inside the autolysosome gets degraded, autolysosomes disintegrate, allowing the recycling of lysosomal membrane proteins and the regeneration of lysosomes from autolysosomes [3] (Fig. 1).
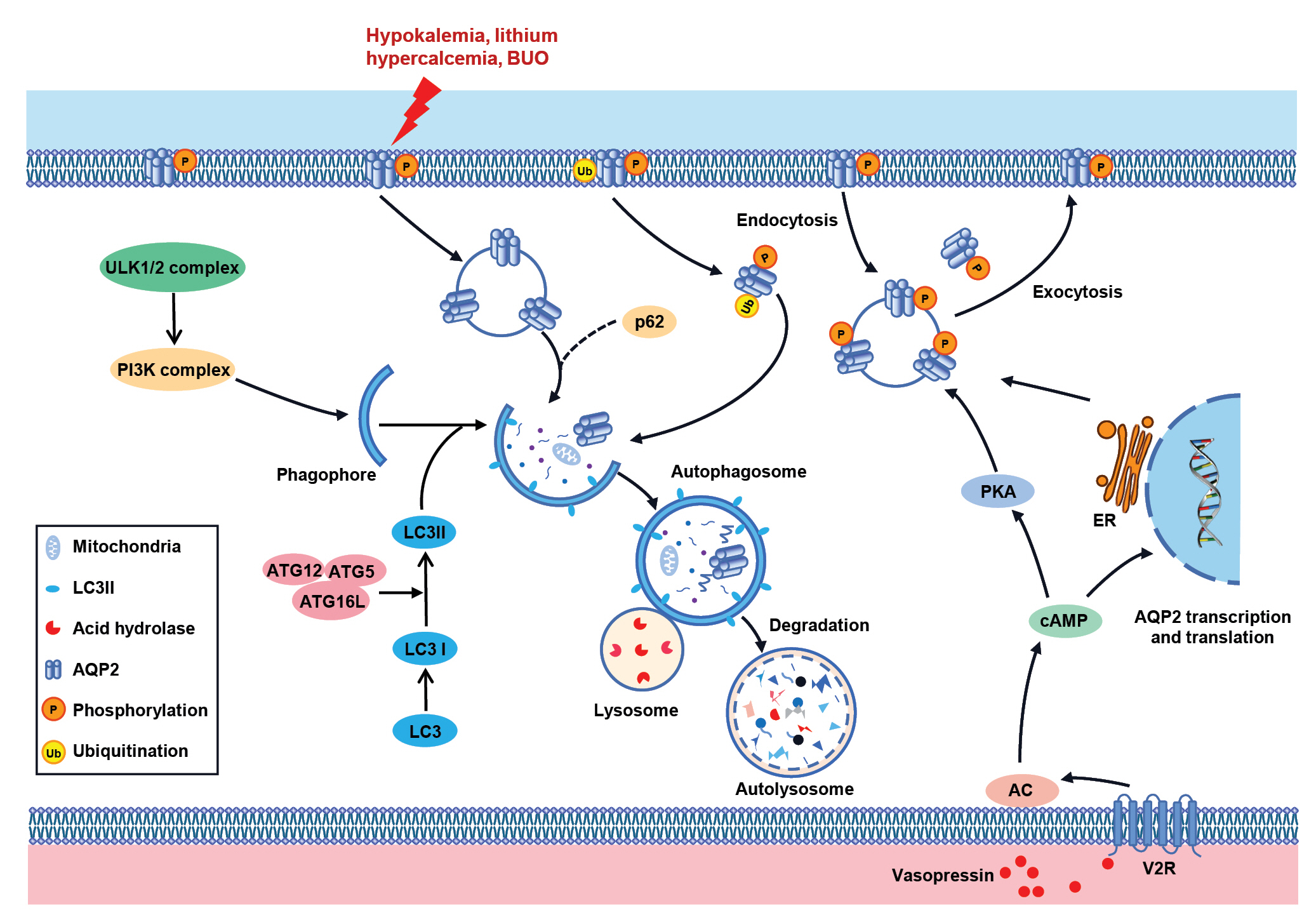
Schematic overview of the regulation of autophagy on AQP2 in the kidney.
The classical autophagy process is initiated by the formation of the ULK1/2 complex, which promotes the phosphorylation of PI3K complex, contributing to the formation of the autophagosome membrane. The expansion and maturation of the autophagosome are completed under the regulation of ATG12–ATG5–ATG16L and LC3 systems. In NDI models like hypokalemia, lithium treatment, hypercalcemia, and BUO models, autophagy is induced in the renal principal cells, causing lysosomal-dependent degradation of AQP2 (left). Protein expression and intracellular trafficking of AQP2 are modulated by arginine vasopressin (AVP) through short- and long-term regulation (right). AVP induces the rapid translocation of vesicles containing AQP2 to the apical membrane in the collecting duct principal cells. When AVP is retrieved, a large fraction of AQP2 returns to the cytoplasm to be recycled or to respond to a new AVP challenge, while other AQP2 is ubiquitinated and then degraded via the lysosomal pathway. AQP2 is subjected to post-translational modifications, including phosphorylation and ubiquitylation, which potentially play key roles in its function. Long-term regulation is based on changes in AQP2 abundance via AVP-induced prolongation of the AQP2 protein half-life and Aqp2 gene transcription (right).
AC, adenylate cyclase; AQP, aquaporin; ATG, autophagy-related gene; BUO, bilateral ureteral obstruction; cAMP, cyclic adenosine monophosphate; ER, endoplasmic reticulum; LC3, light chain 3; NDI, nephrogenic diabetes insipidus; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A.
Microautophagy indicates the process in which cytoplasmic contents are submerged with lysosomes for degradation after lysosome invagination [12]. To date, however, the process of microautophagy and its mechanism have not been thoroughly studied.
CMA is a highly specific form of autophagy that mediates the degradation of soluble cytoplasmic proteins in lysosomes [13]. CMA has only been studied in mammalian cells so far. Unlike macroautophagy and microautophagy, proteins degraded through CMA are recognized by the cytoplasmic chaperone Hsc70 [14]. Hsc70 and accessory partners bring substrates to the lysosome surface. Subsequently, lysosomal membrane protein 2A recognizes and binds to substrate proteins containing the KFERQ pentapeptide sequence and finally completes the degradation of the protein [15].
Autophagy is not always a simple and non-selective cytosolic degradation pathway, and it can be selective to eliminate specific cargoes [16]. For example, mitophagy is the best-studied form of selective autophagy of mitochondria and is chiefly mediated by LC3-associated autophagy receptors by either Ub-independent or Ub-dependent pathways [3].
Besides the autophagy–lysosome pathway, the Ub–proteasome system also contributes to cellular homeostasis. Through this pathway, Ub-tags are added to damaged or unfolded proteins by E3 Ub–ligases and thereby degraded by the proteasome. A fine balance between the Ub–proteasome system and the autophagy–lysosome pathway exists. The use of ubiquitination as a recognition signal indicates a close connection between both pathways [9]. Autophagy seems to degrade larger protein aggregates, while the Ub–proteasome system clears small protein oligomers and misfolded proteins in the cell [9,17].
Dysregulated autophagy has been found to contribute to the pathogenesis of acute kidney injury (AKI), incomplete kidney repair, and chronic kidney diseases, indicating the key roles of autophagy in maintaining kidney homeostasis. However, questions remain as to whether autophagy plays a protective or a pathogenetic role in kidney diseases. The signaling pathways and precise mechanisms underlying autophagy in different types of renal cells and several kidney diseases remain unknown.
Aquaporins in the kidney
Water permeability in the nephron and high medullary interstitial osmolality are important factors for urine concentration [18,19]. Aquaporins (AQPs) are water channel proteins that mediate the osmotic water transport across the cell membrane [20].
Water reabsorption in the kidney is mainly facilitated by AQPs located in the tubular epithelial cells. At least seven AQPs have been found to be expressed in the renal tubule. For example, AQP1 is expressed in the apical and basolateral plasma membranes of the proximal tubule and thin descending limb, while AQP2 is expressed in the principal cells of collecting ducts from the cortex to the inner medulla and undergoes arginine vasopressin (AVP)-regulated water reabsorption. Separately, AQP3 and AQP4, which are expressed at the basolateral membrane of collecting duct principal cells, represent exit pathways for water reabsorbed via AQP2 expression at the apical plasma membrane.
AQP2 is regulated by the anti-diuretic hormone vasopressin on both a short- and long-term basis for water reabsorption in the collecting ducts [21]. Short-term regulation is dependent on the translocation of AQP2-expressing vesicles. AVP induces the rapid translocation of vesicles containing AQP2 to the apical membrane and increases the water permeability in the collecting duct principal cells. When AVP stimulation is retrieved, a large fraction of AQP2 on the plasma membrane can be recycled and participate in a new round of translocation to the apical membrane in response to another AVP challenge, while another fraction of AQP2 is ubiquitinated and degraded via the proteasomal and lysosomal pathways [22]. Long-term regulation is based on the changes in AQP2 abundance via AVP-induced prolongation of the AQP2 protein half-life and Aqp2 gene transcription [23] (Fig. 1).
AQP2 is subjected to posttranslational modifications, including phosphorylation and ubiquitylation, which may play key roles in its function. Intracellular shuttle signaling and protein–protein interaction are associated with AQP2 phosphorylation at several sites in the C-terminus. The vasopressin-dependent activation of cyclic adenosine monophosphate-dependent kinase A (protein kinase A) leads to the phosphorylation of AQP2 at serine 256 (S256), which is considered a priming event for downstream phosphorylation at S269 and S264. Vasopressin stimulation also results in the significant dephosphorylation of AQP2 at S261 [24–26]. There are a trio of putative potential ubiquitination sites (cytosolic lysine residues) within the C-terminal domain of AQP2 (K228, K238, and K270). Among them, K270 is the only substrate for ubiquitination, with 1–3 Ubs added in a K63-linked chain [22,27–30]. The ubiquitination of AQP2 at the plasma membrane promotes AQP2 internalization and subsequent proteasomal degradation [22]. Phosphorylation at S261 follows ubiquitination and may stabilize AQP2 intracellularly [31].
Dysregulation of AQP2 has been demonstrated to play a role in water balance disorders, e.g., nephrogenic diabetes insipidus (NDI) and systemic water retention [32]. NDI is triggered by the kidney’s incapability to respond to vasopressin stimulation, leading to polyuria and polydipsia [33]. Compared to the inherited type of NDI, acquired forms of NDI are much more common, among which electrolyte disorders (hypokalemia and hypercalcemia), ureteral obstruction, and lithium use are associated with reduced messenger RNA and protein expression of AQP2 in the kidney [19,34]. The precise underlying molecular mechanisms of these types of NDI remain to be resolved. However, emerging data imply the role of autophagy in the degradation of AQP2 protein during NDI (Table 1).
Autophagy-induced degradation of aquaporin 2 in nephrogenic diabetes insipidus
Electrolyte imbalances, like hypokalemia and hypercalcemia, are a relatively common kind of electrolyte disorder clinically. Both hypokalemia and hypercalcemia can cause mild NDI characterized by reduced AQP2 protein expression in the collecting duct principal cells [35,36]. Recent studies have demonstrated that AQP2 downregulation seen in hypokalemia and hypercalcemia may be mediated by autophagic degradation [37–39] (Fig. 1).
By mass spectrometry (MS)-based proteomics and liquid chromatography-MS/MS, Khositseth et al. [38] demonstrated a reduction in the abundance of AQP2 in the inner medullary collecting ducts (IMCD) and proteins associated with energy metabolism in mitochondria, the organization of the actin cytoskeleton, and cellular adhesion in hypokalemic rats. AQP2 phosphorylation at both S256 and S261 was also downregulated. Under electron microscopy, single-membrane autophagolysosomes containing electron-dense membrane structures and double-membrane autophagosome vesicles or phagophores were identified in IMCD cells of the potassium-deprived rats. Immunofluorescence and double-label immunogold electron microscopy revealed the co-localization of AQP2 with both autophagosomes (LC3) and lysosome (Lamp1) in the IMCD cells of the potassium-deprived rats, supporting the role of autophagic protein degradation as the mechanism involved in the downregulation of AQP2 in IMCD cells after hypokalemia. Interestingly, after potassium replenishment, the normal phenotype of IMCD cells was restored, which coincided with the normalization of AQP2 levels, and its colocalization with autophagy markers was abolished. This indicates that autophagic degradation at least partially contributes to AQP2 downregulation during hypokalemia [38].
Ideally, setting up hypokalemia models in animals with knockdown of various ATGs may be an excellent way to better understand the role of autophagy in AQP2 regulation [38]. Kim et al. [39] generated conditional knockout mice in whom ATG7, a catalyst in autophagy conjugation systems and autophagosome formation, was genetically ablated specifically in AQP2-positive principal cells (Atg7Δpc) of the collecting duct. Thereafter, the group investigated AQP2 regulation in hypokalemia in these mice, and their findings support the role of autophagy in the degradation of AQP2 and further indicate the importance of the canonical autophagy pathway for AQP2 regulation in hypokalemia.
Consumption of a low-K+ diet for 2 weeks caused a significant polyuria and urine concentration defect in both Atg7f/f and Atg7Δpc mice, with these changes being much more pronounced in the Atg7Δpc mice compared to the Atg7f/f mice. Consistent with this, the protein abundance and apical expression of both AQP2 and pS256-AQP2 in IMCD cells were markedly reduced in both strains, albeit again more so in Atg7Δpc mice compared to Atg7f/f mice. Notably, after K+-depletion, in Atg7f/f mice, pS261-AQP2 was located restrictively in the intracellular vesicles, while, in Atg7Δpc mice, pS261-AQP2 was found diffusely throughout the cytoplasm, indicating a block of pS261-AQP2 internalization in IMCD cells. In Atg7Δpc mice, in contrast to the reduction of AQP2 protein expression, urinary AQP2 excretion was significantly increased after hypokalemia compared to in Atg7f/f mice [39].
As expected, hypokalemia induced LC3-positive canonical autophagy in the IMCD cells of K+-depleted Atg7f/f mice, while, in K+-depleted Atg7Δpc mice, LC3-negative noncanonical autophagy seemed to have occurred, as more LC3-negative vacuoles were seen in IMCD cells. Interestingly, in K+-depleted Atg7f/f mice, pS261-AQP2 and LC3 were colocalized with LC3-positive puncta, which were confirmed by immunoelectron microscopy to be large irregular-shaped autophagic vacuoles. Surprisingly, in K+-depleted Atg7Δpc mice, pS261-AQP2 immunolabeling was not detected in the small, round autophagic vacuoles (non-canonical autophagic vacuoles), indicating that pS261-AQP2 is subject to the same degradation pathway as total AQP2 in hypokalemia. A large intracellular accumulation of pS261-AQP2 in concert with ATG7 deletion may be attributed to the impairment of other compensatory lysosomal-degradation mechanisms by inactivated lysosomes [39].
Khositseth et al. [37] further investigated the role of autophagy in NDI induced by hypercalcemia. Similar to hypokalemia, autophagy was responsible for AQP2 downregulation in IMCDs and, thereby, the urine concentration defects in hypercalcemia induced by either vitamin D or parathyroid hormone.
Unilateral ureteral obstruction and bilateral ureteral obstruction (BUO) or release are associated with decreased levels of water channel proteins, including AQP2 in the collecting ducts, which leads to impaired urinary concentrations [40]. In early BUO, AQP2 and pS261-AQP2 are redistributed more intracellularly and in a more clustered fashion. They are also colocalized with early endosomes and lysosomes, suggesting an early downregulation of AQP2 likely through a lysosomal-degradation pathway [41]. Supporting the increased degradation of AQP2 after ureteral obstruction, a recent study showed that, in early BUO, AQP2 was degraded by the autophagy pathway together with some other critical proteins and damaged organelles [42]. However, whether pharmacological or genetic inhibition of autophagy prevents the downregulation of AQP2 and improves NDI was not examined in these studies.
Lithium treatment results in a downregulation of AQP2 with consequent polyuria in vivo [43,44]. However, the molecular mechanisms underlying this remain unclear. Proteomics [45] and RNA sequencing [46] demonstrated that several signaling pathways are activated by lithium treatment, such as gene expression, cytoskeletal organization, apoptosis, cell proliferation [45], and an inflammatory-like response [46]. Lithium-induced lysosomal degradation through glycogen synthase kinase-3β (GSK3β) [47] or autophagy-lysosomal degradation through SNX27 might play a role in the downregulation of AQP2 [48]. Lithium was actually considered a potent autophagy inducer, and its autophagy-enhancing property likely contributes to the therapeutic benefit of patients with neuropsychiatric disorders [49]. Interestingly, chloroquine, an autophagy inhibitor that acts by impairing autophagosome fusion with lysosomes, mitigates the lithium-induced downregulation of AQP2 and polyuria. A study from our laboratory showed that lithium treatment induced ER stress in IMCD cells, and the attenuation of ER stress by chaperon improved lithium-induced NDI [50]. Interestingly, a remarkable characteristic in BUO is the presence of ER stress together with autophagy in IMCD cells [42]. It is known that sustained and unsolved ER stress can cause autophagy [51]. Whether the inhibition of ER stress prevents autophagy and improves NDI has not yet been examined.
The role of autophagy in NDI is important, yet a few questions remain. First, is the degradation of AQP2 in the NDI specific? In hypercalcemia, in addition to AQP2, proteins involved in regulating actin filament polymerization, cytoskeletal protein binding, and cell–cell junctions are also downregulated, which are associated with the disruption of cell–cell junctions between IMCD cells and disorganized actin filaments at tight junctions [37]. These histopathological changes are unfavorable for AQP2 intracellular trafficking and expression. In contrast, in hypokalemia, the presence of damaged mitochondria and mitophagy in IMCD was clearly observed in addition to decreased cytoskeletal protein levels, indicating that intracellular K+ depletion may alter mitochondrial function, decrease adenosine triphosphate production, and promote the generation of reactive oxygen species (ROS), which are supposed to cause damage to IMCD cells. Secondly, why does autophagy occur in NDI? Is autophagy a friend or a foe? Autophagy can be protective or can cause damage, depending on the specific disease stage and cell types. The initiation of autophagy in the early period of NDI may be beneficial for maintaining cell homeostasis when tubular cells face stress coming from electrolyte imbalance, ROS, etc. Autophagy may be required for tubular cells to discontinue tubular transport and to decrease energy consumption for survival, as reviewed recently [52]. However, if autophagy in NDI is not specific to target AQPs (and/or channels, transporters), extensive apoptosis and cell death will be unavoidable. The next question will be whether suppression of autophagy improves NDI. Theoretically, autophagy inhibition increases AQP2 protein expression and improves urine concentration, as seen in a prior lithium study [53]. Anti-autophagy is not simple, and targeting autophagy as a therapeutic intervention may be challenging. Nonspecific inhibitors could have effects other than autophagy inhibition. Interfering with autophagy may ameliorate clinical symptoms for a short time, but the outcome will need to be carefully evaluated in the long term.
Regulation of aquaporin 1 by autophagy in the kidney
Renal ischemia/reperfusion (I/R) is the main cause of AKI and contributes to high morbidity and mortality rates. Early studies have demonstrated that I/R-induced AKI is associated with reduced AQPs protein expression [54,55]. The role of autophagy in kidney I/R injury—in particular, in proximal tubules—has been extensively investigated. The induction of autophagy in kidney tubular cells, particularly in proximal tubular epithelial cells, has been documented in rodent models of I/R-induced AKI [3], although suppressed autophagy has also been observed [56,57]. Our recent study revealed that the induction of autophagy by TDZD-8 (a GSK-3β inhibitor) after I/R can rescue reduced AQP1 protein expression, likely by way of clearing deleterious factors (e.g., interleukin-1β) [58]. GSK-3β is known to modulate autophagy. GSK-3β overexpression activates the mammalian target of rapamycin complex 1 and suppresses autophagy, while its inhibition with inhibitors increases autophagic flux [59]. In vitro hypoxia/reoxygenation (H/R) induced suppression of autophagy and downregulation of AQP1 in murine IMCD 3 (mIMCD3) cells expressing endogenous AQP1, which was fully prevented by TDZD-8 [58]. Interestingly, the inhibition of autophagy by 3-methyladenine or Atg5 gene knockdown attenuated the recovery of AQP1 protein expression induced by TDZD-8 in mIMCD3 cells with H/R. The activation of autophagy by TDZD-8 can also inhibit the activation of the NLR family pyrin domain containing 3 inflammasome. Therefore, it is believed that autophagy mediates the removal of protein aggregates, damaged organelles, and deleterious molecules to maintain cellular homeostasis, which might protect cells and tissues against injury.
In mammals, the renal medulla is constantly under hypertonic conditions. This extreme hyperosmotic condition is generally harmful to other cells, and renal medullary cells must make themselves adapt to this harsh environment and survive and function normally. In one study, mIMCD3 cell line was derived from the terminal IMCDs of mice transgenic for the early region of simian virus SV40 [60]. It is highly useful for the study of cellular adaptation to osmotic stress and the transport physiology of this nephron segment. Our unpublished data showed that hypoosmotic stress (300 mOsm) activates autophagy and downregulates AQP1 expression, which can be prevented by the inhibition of autophagy with 3-methyladenine, chloroquine, or Atg gene knockdown (unpublished data). Taken together, the activation of autophagy regulates AQP1 expression in both renal injury and physiological conditions.
In the kidney, AQP3 and AQP4 are expressed at the basolateral membrane of collecting duct principal cells [61–63], AQP6 is found in intercalated cells in the collecting ducts [64], AQP7 is located in the proximal tubules [65], and AQP11 is expressed intracellularly in the proximal tubules and identified in the ER [66]. So far, whether the degradation of these AQPs is associated with autophagy is still unknown, and more studies are required.
Perspectives
Emerging evidence has demonstrated that autophagy plays a key role in AQPs regulation in the kidney. Autophagy contributes to the downregulation of AQPs in several animal models of NDI, although its significance is unclear. The modulation of autophagy might provide an option to improve types of water balance disorders. One would suppose that adequate induction of autophagy might reduce water reabsorption in the kidney, which may prevent water retention. To accomplish this, it is crucial to clarify the mechanisms of autophagy induction in tubular cells and define the underlying signaling pathways and the resulting roles played by autophagy. The other noteworthy question is whether autophagy is regulated by the altered expression of AQPs in the kidney. AQP11 null mice exhibit the phenotype of rapid polycyst development in association with stimulated autophagy with enhanced apoptosis and ER stress before and after cyst formation [67,68]. Autophagy is likely induced by ER stress in this condition; however, autophagy could also cause ER stress and apoptosis [69]. This finding indicates, at least partly, that the conditions provoked by AQP11 deficiency regulate autophagy. In addition, our unpublished data suggest that either knockdown or inhibition of AQP1 suppresses autophagy induced by rapamycin in mIMCD3 cells (unpublished data). More studies are required to define the physiological role of AQPs in autophagy, which would provide insights and an understanding of the regulatory mechanism of autophagy.
Notes
Conflicts of interest
Tae-Hwan Kwon is the Associate Editors of Kidney Research and Clinical Practice and were not involved in the review process of this article. All authors have no other conflicts of interest to declare.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 81870465 and 82170693); a National Research Foundation of Korea (NRF) grant funded by the Korean government (MIST) (grant no. 2021R1A5A2021614); and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (grant no. HI15C0001 to THK).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: YK, THK, CL, WW
Funding acquisition, Supervision: WW
Writing–original draft: XG
Writing–review & editing: YK, THK, CL, WW
All authors read and approved the final manuscript.

