| Kidney Res Clin Pract > Volume 38(2); 2019 > Article |
|
Abstract
Background
Methods
Results
Notes
Authors’ contributions
Hyang Ki Min and Sung Woo Lee participated in the research ideation, study design, and drafting the article. Su Ah Sung and So Young Lee participated in the data analysis/interpretation and statistical analysis. So Young Lee and Sung Woo Lee participated in the supervision or mentorship. All authors read and approved the final manuscript.
Figure 1
GAM plot between urine SGU and eGFR
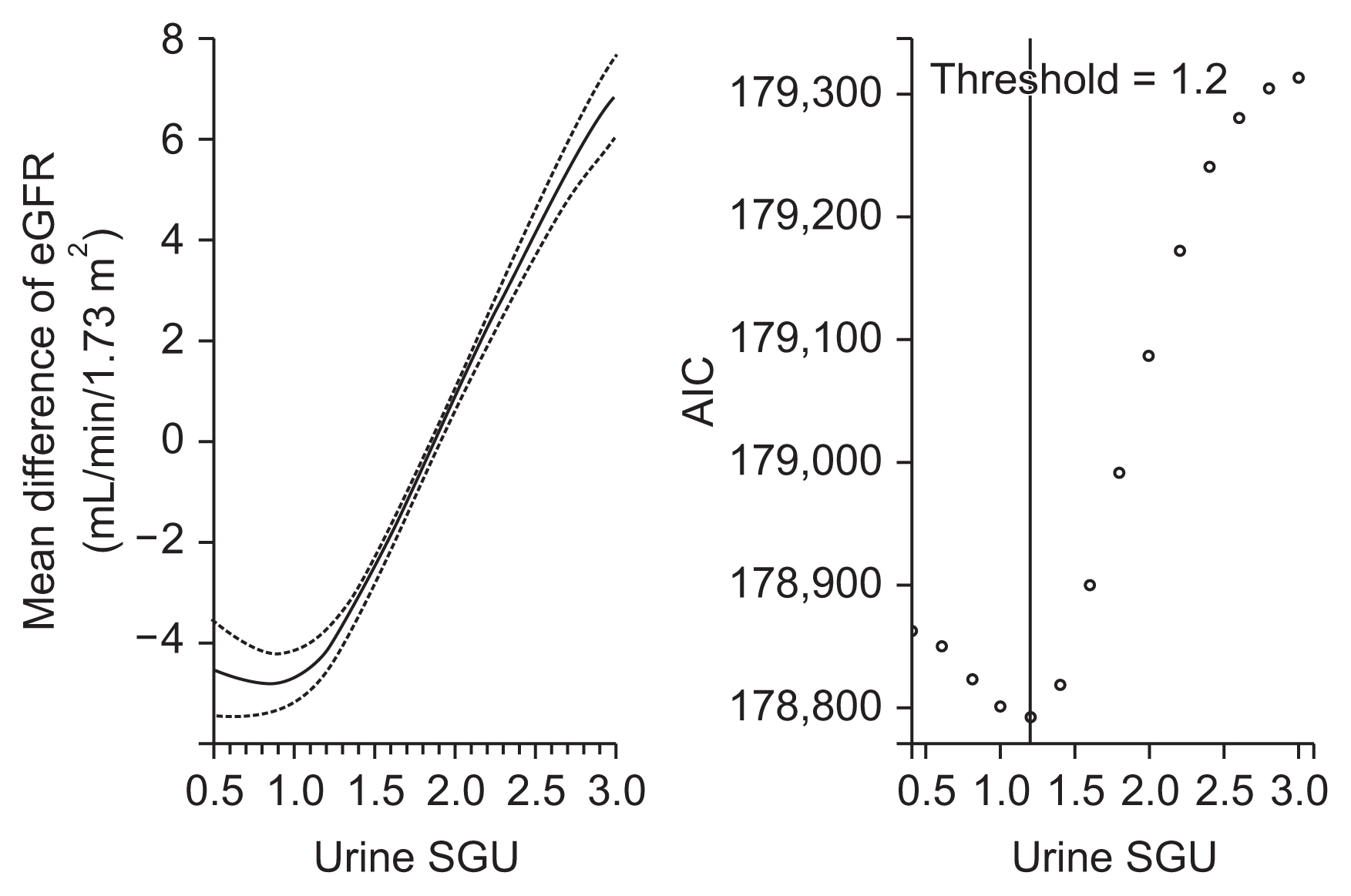
Figure 2
Odds ratio (OR) of urine SGU quartiles for high eGFR

Figure 3
Penalized smoothing splines showing the relationship between urine SGU and high eGFR
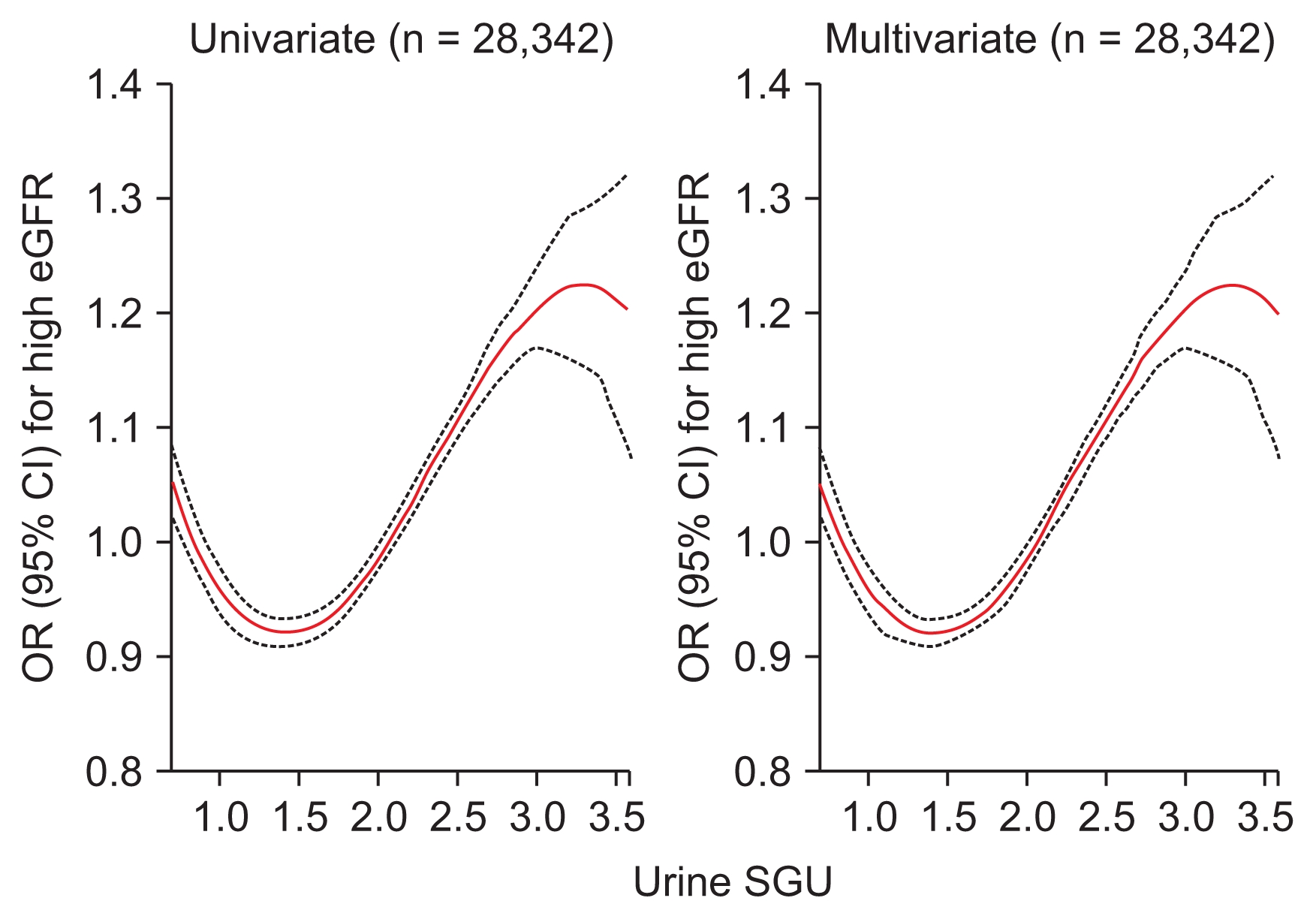
Table 1
| Characteristic | Quartile of urine SGU | P-trend | |||
|---|---|---|---|---|---|
|
|
|||||
| < 1.4 (n = 5,261) | 1.4 to 1.8 (n = 6,355) | 1.8 to 2.2 (n = 6,988) | ≥2.2 (n = 9,738) | ||
| Age (yr) | 53.8 ± 15.5 | 55.1 ± 15.5a | 52.1 ± 15.4a,b | 44.9 ± 15.4a,b,c | < 0.001 |
| Sex, male (%) | 29.9 | 40.4a | 48.1a,b | 54.6a,b,c | < 0.001 |
| Current smoker (%) | 15.2 | 17.1a | 22.3a,b | 26.9a,b,c | < 0.001 |
| Frequent drinker (%) | 16.0 | 19.5a | 25.2a,b | 24.9a,b | < 0.001 |
| Regular walking (%) | 27.0 | 26.6 | 26.5 | 27.0 | 0.820 |
| Hypertension (%) | 38.0 | 40.6a | 34.4a,b | 26.1a,b,c | < 0.001 |
| Diabetes (%) | 12.6 | 13.3 | 10.3a,b | 9.9a,b | < 0.001 |
| Dyslipidemia (%) | 20.8 | 21.4 | 19.1a,b | 15.8a,b,c | < 0.001 |
| Cardiovascular disease (%) | 5.0 | 5.6 | 4.7b | 2.8a,b,c | < 0.001 |
| BMI (kg/m2) | 23.5 ± 3.2 | 23.8 ± 3.3a | 23.8 ± 3.4a | 23.9 ± 3.5a | 0.008 |
| Waist circumference (cm) | 80.8 ± 9.5 | 82.0 ± 9.7a | 82.1 ± 9.8a | 81.8 ± 10.3a | 0.025 |
| Systolic BP (mmHg) | 122.3 ± 18.9 | 123.0 ± 18.6 | 120.7 ± 16.8a,b | 117.1 ± 15.5a,b,c | < 0.001 |
| Diastolic BP (mmHg) | 76.2 ± 10.7 | 76.8 ± 10.7 | 76.8 ± 10.4 | 76.3 ± 10.3 | 0.608 |
| Pulse rate (counts/min) | 69.9 ± 9.2 | 69.9 ± 9.3 | 70.1 ± 9.3 | 70.4 ± 9.8 | 0.002 |
| Fasting glucose (mmol/L) | 5.44 ± 1.11 | 5.50 ± 1.12 | 5.46 ± 1.11 | 5.54 ± 1.60a,c | < 0.001 |
| Total cholesterol (mmol/L) | 4.90 ± 0.93 | 4.91 ± 0.94 | 4.93 ± 0.93 | 4.88 ± 0.93c | 0.488 |
| eGFR (mL/min/1.73 m2) | 92.9 ± 18.2 | 91.0 ± 17.9a | 93.7 ± 16.3b | 98.4 ± 15.9a,b,c | < 0.001 |
| ≥ 90 (%) | 60.3 | 55.0a | 61.3b | 70.9a,b,c | < 0.001 |
| 60–90 (%) | 34.7 | 39.5a | 35.7b | 27.8a,b,c | < 0.001 |
| 30–60 (%) | 4.4 | 5.2a | 2.9a,b | 1.3a,b,c | < 0.001 |
| < 30 (%) | 0.6 | 0.3a | 0.1a,b | 0.0a,b,c | < 0.001 |
| BUN (mmol/L) | 4.7 ± 1.8 | 5.1 ± 1.5a | 5.2 ± 1.5a,b | 5.5 ± 1.5a,b,c | < 0.001 |
| Serum creatinine (μmol/L) | 71.2 ± 30.5 | 73.4 ± 23.3a | 73.4 ± 16.4a | 74.5 ± 15.3a,b,c | < 0.001 |
| Urine SGU | 1.0 ± 0.2 | 1.5 ± 0.1a | 1.9 ± 0.1a,b | 2.5 ± 0.3a,b,c | < 0.001 |
| Urine sodium (mmol/L) | 81.1 ± 36.3 | 123.3 ± 42.6a | 140.0 ± 48.6a,b | 137.7 ± 51.9a,b | < 0.001 |
| Urine creatinine (mmol/L) | 5.1 ± 2.1 | 9.0 ± 2.9a | 13.0 ± 4.0a,b | 19.6 ± 6.9a,b,c | < 0.001 |
| Urine sodium to creatinine | 18.7 ± 11.9 | 15.7 ± 8.7a | 12.3 ± 7.3a,b | 8.3 ± 5.1a,b,c | < 0.001 |
| High water intake (%) | 34.4 | 29.6a | 27.6a | 27.9a,b | < 0.001 |
| Proteinuria (%) | 0.7 | 1.4a | 1.1a,b | 1.4a | 0.002 |
Data are presented as mean ± standard deviation for continuous variables and a percentage for categorical variables.
Conversion factor from conversional unit to SI unit was in × 0.0555 in glucose, × 0.0259 in cholesterol, × 0.357 in BUN, and × 88.4 in serum and urine creatinine, respectively.
BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; SGU, specific gravity unit.
Table 2
Table 3
Beta and 95% confidence interval (CI) were analyzed using multivariate linear regression analysis with the following covariates: systolic and diastolic blood pressure (BP), pulse rate, fasting glucose, total cholesterol, waist circumference, age, sex, proteinuria, cardiovascular disease, smoking and drinking status, high water intake, and urine sodium and creatinine. When covariates were chosen as dependent variables, they were excluded from the model.
References
- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print
















