| Kidney Res Clin Pract > Volume 37(2); 2018 > Article |
|
Abstract
Background
Major adverse cardiac and cerebrovascular events (MACCEs) are main concerns in patients with atrial fibrillation (AF); however, factors affecting MACCEs remain inconclusive in AF patients chronically treated with digoxin. We investigated the major clinical determinants for fatal MACCEs in AF patients treated with digoxin over a 10-year follow-up period.
Methods
We analyzed a retrospective cohort of 1,480 AF patients at Eulji University Hospital, Daejeon, South Korea from March 2004 to August 2015. Among this population, 402 consecutive patients receiving chronic digoxin therapy were selected for the study. Data for electrocardiography, medication history, laboratory values including the serum digoxin concentration (SDC) and fatal MACCEs were collected. All data were divided and compared between groups based on the occurrence of MACCEs.
Results
The overall incidence of fatal MACCEs among the 402 digoxin-treated AF patients (age, 68 ± 11 years; male, 40.3%) was 12.1%. These fatalities resulted from heart failure (46.1%), fatal stroke (26.9%), fatal myocardial infarction (15.3%) and sudden cardiac death (5.7%). A higher prevalence of diabetes, pre-existing ischemic heart disease (IHD), lower estimated glomerular filtration rate (eGFR), higher SDC, and junctional bradycardia were more frequently observed in patients with MACCEs compared to those without MACCEs. Multivariable analysis showed that an eGFR of ≤ 60 mL/min/1.73 m2 and pre-existing IHD had a hazard ratio of 3.35 and a confidence interval of 1.64–6.87 (P < 0.001) for fatal MACCEs.
Rate control in atrial fibrillation (AF) patients who are refractory to or unable to take β-blockers or non-hydropyridine calcium channel blockers (CCBs) remains a significant concern. Although there is increase awareness about the potential pro-arrhythmic properties and toxicity of digoxin [1], this medication has been widely prescribed for decades and is recommended for rate control in AF, regardless of the patient’s renal function or presence of structural heart disease [2–4]. Non-randomized analyses of data from a large AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial [5,6] and other AF-related trials have shown a higher mortality associated with chronic digoxin treatment. Real-world data from large AF cohorts from the veterans administration [7] and health maintenance organizations [8] have also confirmed these findings. Contemporary literature suggests that chronic digoxin treatment is independently associated with a greater risk of cardiac and cerebrovascular mortality, which includes hospitalization [8,9] and stroke in patients with AF [10]. In addition, this mortality risk seems to increase in digoxin-treated AF patients with pre-existing ischemic heart disease (IHD) [7,11]. However, in the MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherap) trial, digoxin treatment was not associated with a significant increase in cardiac mortality over the course of a one-month follow-up [12]. Because there is limited long-term data on the major determinants for digoxin-associated mortality in AF patients, we performed a retrospective analysis of a large cohort of AF patients and clarified the major clinical determinants of cardiac and cerebrovascular mortality in AF patients chronically treated with digoxin.
From a total AF cohort of 1,480 patients, we retrospectively analyzed 402 consecutive digoxin-treated AF patients who were treated at the tertiary referral center of Eulji University Hospital, Daejeon, South Korea from March 2004 to August 2015. We use electronic medical records from the departments of cardiology, nephrology and neurology to obtain demographic, outpatient and in-patient medication data and intermittent serum digoxin concentrations (SDCs). Patients who were 18 years of age or older with electrocardiographically confirmed AF were included in the study. We considered the date of the patient’s first admission or first visit to an outpatient clinic with a diagnosis of AF as the index digoxin prescription as the date of entrance into the study. Patients who were excluded from the study included those with transient AF, those who were likely to have perioperative AF (defined as having cardiopulmonary surgery), and those with hypothyroidism or hyperthyroidism within three months before AF treatment.
The protocol was approved by the Institutional Review Board (IRB) of Eulji University Hospital (IRB number: 2018-03-118). The study protocol was in accordance with the ethical standards of the IRB of Eulji University Hospital and with the Declaration of Helsinki.
We estimated the glomerular filtration rate (eGFR, mL/min/1.73 m2) using the Modification of Diet in Renal Disease (MDRD) equation and stratified chronic kidney disease (CKD) into five stages [13,14]. IHD was defined as obstructive coronary artery disease with an increase in supply/demand mismatch precipitated by physical or emotional stress or concurrent illness in the documented computer tomography or coronary angiogram [15]. Heart failure was defined as a clinical syndrome that results from decreased systolic function with a documented decreased ejection fraction [16]. We collected data on prescriptions filled for digoxin, β-blockers, non-dihydropyridine CCBs, rhythm control drugs (flecainide, propafenone and amiodarone), diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, aspirin, clopidogrel, and anticoagulants, as well as data for major adverse cardiac and cerebrovascular events (MACCEs) during the study follow-up period. Fatal MACCEs, the primary outcome, were defined as death from heart failure, fatal myocardial infarction, fatal stroke and sudden cardiac death during the follow-up period.
We compared demographic data including underlying disease history, laboratory data, electrocardiogram, and medication history according to the occurrence of MACCEs during the study period. Continuous variables are expressed as means with standard deviations, and categorical variables are expressed as percentages. The independent sample t test and Pearson’s chi-square test were used to compare normally distributed continuous variables and dichotomous variables, respectively. Cox regression hazard models were used to determine independent predictors for the occurrence of MACCEs. Multivariable analysis was adjusted for age, sex, diabetes, CHA2DS2-VASc, eGFR, and IHD. Patients were categorized into four groups according to pre-existing IHD and cut-off eGFR values of 60 mL/min/1.73 m2 in order to characterize the association between each group and MACCEs. A Kaplan-Meier Curve was plotted for the free-from MACCEs. Analyses were performed using MedCalc software (version 17.0; Ostend, Belgium). P values less than 0.05 were considered to be statistically significant.
In our study, 402 (27.2%) consecutive AF patients who were treated with digoxin at the time of prescription index and who had a median a follow-up of 19.0 months (interquartile range, 2.6–44.8 months) were enrolled from the divisions of nephrology, cardiology and neurology. The overall incidence of MACCEs was 12.1% for an average three-year follow-up. The MACCEs consisted of death from heart failure (46.1%), fatal stroke (26.9%), fatal myocardial infarction (15.3%) and sudden cardiac death from fatal ventricular arrhythmia (5.7%). Table 1 lists the baseline characteristics of patients with and without MACCEs in this study. The group with MACCEs had a significantly higher prevalence of diabetes (36.5% vs. 22.3%), pre-existing IHD (57.7% vs. 31.7%), lower eGFR (60.0 ± 35.3 mL/min/1.73 m2 vs. 78.3 ± 35.0 mL/min/1.73 m2), and higher SDC (1.21 ± 0.88 ng/dL vs. 0.93 ± 0.81 ng/dL) when compared to those without MACCEs (Table 2).
Receiver operating characteristic (ROC) analysis showed that pre-existing renal dysfunction (cut-off: eGFR ≤ 60 mL/min/1.73 m2, CKD III–V) significantly predicted the occurrence of MACCEs with a sensitivity of 59% and a specificity of 66% (P = 0.001). We also stratified the AF group into both eGFR ≤ 60 mL/min/1.73 m2 (CKD III–V) and eGFR > 60 mL/min/1.73 m2 (CKD I–II) subgroups according to CKD staging by a nephrologist [17,18]. The AF group with renal dysfunction (eGFR ≤ 60 mL/min/1.73 m2, CKD III–V) also had a larger proportion of males, diabetes and a higher CHA2DS2-Vasc than the group with-out renal dysfunction (CKD I–II) (Supplementary Table 1–3). Electrocardiogram data showed that AF patients with MACCEs had more frequent junctional bradycardia in the emergency room or intensive care unit than those without MACCEs (Table 1). The overall incidence of MACCEs was higher in patients with pre-existing IHD and renal dysfunction (eGFR ≤ 60 mL/min/1.73 m2) than in the AF group without pre-existing IHD and renal dysfunction.
In the Cox proportional hazards regression models, univariate analysis showed that CHA2DS2-VASc had a hazard ratio (HR) of 1.33 and a 95% confidence interval (95% CI) of 1.04–1.69 (P = 0.020). An eGFR ≤ 60 mL/min/1.73 m2 had a HR of 1.57 and a 95% CI of 1.27–3.47 (P = 0.001), and pre-existing IHD had a HR of 1.46 and a 95% CI of 1.21–3.27 (P = 0.001). However, multivariate analysis showed that pre-existing IHD and an eGFR ≤ 60 mL/min/1.73 m2 were significantly associated with MAC-CEs. We then grouped subjects into four categories: 1) pre-existing IHD(−) and eGFR > 60 mL/min/1.73 m2; 2) pre-existing IHD(+) and eGFR > 60 mL/min/1.73 m2; 3) pre-existing IHD(−) and eGFR ≤ 60 mL/min/1.73 m2; and 4) pre-existing IHD(+) and eGFR ≤ 60 mL/min/1.73 m2 (Table 3). The Kaplan-Meier curve showed that concurrent pre-existing IHD with an eGFR ≤ 60 mL/min/1.73 m2 was significantly associated with a lower MACCE-free survival during the 10-year follow-up (Fig. 1). In multivariable analysis, concurrent pre-existing IHD with an eGFR ≤ 60 mL/min/1.73 m2 was significantly associated with a higher risk of MACCEs (HR, 3.35; 95% CI, 1.64–6.87; P < 0.001) (Table 3).
In our study, data from AF patients receiving chronic digoxin treatment were collected, and the overall incidence of MACCEs was found to be 12.1% during the follow-up period. AF patients with fatal MACCEs had a significantly larger proportion of pre-existing IHD and renal dysfunction than those without fatal MACCEs. More specifically, concurrent renal dysfunction (cut-off: eGFR ≤ 60 mL/min/1.73 m2; CKD III–V) with pre-existing IHD was a major determinant of fatal MACCEs.
Present guidelines recommend digoxin as a rate control option in patients with AF, regardless of renal dysfunction or IHD [4]. It has been used in AF patients who were unsuitable for or who had poor compliance with β-blockers and non-dihydropyridine CCBs. The digoxin maintenance dose (0.125–0.25 mg daily) and its adjustment are recommended for patients with renal dysfunction, as CKD can reduce the drug’s excretion [19,20]. The monitoring of SDC is warranted in renal dysfunction patients with chronic digoxin treatment.
Recently, digoxin was shown to be significantly associated with an increased mortality risk in patients with AF [8,9,21], and previous results raised the question of what is the determinant for digoxin-related mortality in AF patients with chronic digoxin treatment. An AFFIRM secondary analysis showed that digoxin treatments were associated with all-cause and cardiac mortality [5]. AF patients treated with digoxin for six years had a higher proportion of pre-existing IHD than those not treated with digoxin, and the results showed an all-cause mortality with a HR of 1.41 and a 95% CI from 1.19 to 1.67. The study also showed cardiovascular mortality with a HR of 1.35 and a 95% CI from 1.06 to 1.71 and an arrhythmic mortality with a HR of 1.61 and a 95% CI from 1.12 to 2.30. In our study, digoxin-treated AF patients with MAC-CEs had a higher proportion of diabetes, pre-existing IHD and renal dysfunction than those without MACCEs, as shown in Table 1. In the multivariate Cox regression analysis, our study also showed that pre-existing IHD (HR, 1.21; 95% CI, 1.17–2.78; P = 0.011) and an eGFR ≤ 60 mL/min/1.73 m2 (HR, 1.27; 95% CI, 1.14–2.78; P = 0.016) were significantly associated with an increased risk of fatal MACCEs (Table 3).
The Digitalis Investigation Group trial showed that digoxin reduced hospitalization but not fatal MACCEs when compared with a placebo [22]. However, this post-hoc analysis was limited by its exclusion of AF patients at baseline [23] and by the lack of data on digoxin doses or the SDC, which were also not collected in the ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) [24]. In our study, we collected and analyzed the digoxin maintenance dose and SDC in digoxin-treated AF patients. In addition, the digoxin maintenance dose (0.17 ± 0.15 mg vs. 0.15 ± 0.05 mg) was similarly prescribed between the AF patients with and without MACCEs. However, the SDC in the AF patients with MACCEs was higher than in those without MACCEs (1.21 ± 0.88 ng/dL vs. 0.93 ± 0.81 ng/dL, P = 0.023), which suggests that digoxin toxicity is significantly associated with the occurrence of fatal MACCEs in digoxin-treated AF patients. Our study also showed the association between the prescribed digoxin maintenance dose and digoxin-related fatal MACCEs in clinical practice.
In the post-hoc analysis from the ROCKET AF [24], digoxin treatment was used frequently in baseline AF (68%, male; average CHAD2 3), and it was significantly associated with an increased risk of all-cause mortality (HR, 1.17; 95% CI, 1.04–1.32; P = 0.0093), vascular death (HR, 1.19; 95% CI, 1.03–1.39; P = 0.0201), and sudden death (HR, 1.36; 95% CI, 1.08–1.70; P = 0.0076). For rate control in AF patients being treated with other medications such as β-blockers (64%) and CCBs (25%), the findings of this study suggest that digoxin should not be a first-line therapy because it influenced the incidence of the MACCEs during the follow-up. In our study, β-blockers (30.8%) and non-dihydropyridine CCBs (15.4%) were less frequently used compared with a previous study. The effect of digoxin as monotherapy could minimize the effect of β-blockers and CCBs, which influence the incidence of MACCEs.
In the TREAT-AF study [7], the proportion of those receiving digoxin treatment was 98.5% for males, 93.1% for those with a CHAD2 score of 0 to 3, and 4.8% for those with pre-existing IHD and an average eGFR of 67 mL/min/1.73 m2. There was evidence of increased risk in patients with a pre-existing IHD (HR 1.49, 95% CI 1.34–1.67, and P = 0.002 in the full cohort; HR 1.45, 95% CI 1.26–1.66, and P = 0.077 in the propensity-matched cohort). In addition, with multivariate adjustment and propensity matching, digoxin was significantly associated with an increased risk of death among all strata of GFR, except in dialysis patients. However, there was still no evidence of effect modification in all strata of kidney function. In our study, we stratified the AF group into both eGFR ≤60 mL/min/1.73 m2 (CKD III–V) and eGFR > 60 mL/min/1.73 m2 by ROC analysis in accordance with CKD management guidelines [17,18]. In multi-variable Cox regression analysis, pre-existing IHD(−) and eGFR > 60 mL/min/1.73 m2 was used as a categorical reference, and pre-existing IHD(+) and an eGFR ≤ 60 mL/min/1.73 m2 had a HR of 3.35 with a 95% CI of 1.64–6.87 (P < 0.001). In the Kaplan-Meier analysis, pre-existing IHD(+) and an eGFR ≤ 60 mL/min/1.73 m2 was significantly associated with the occurrence of MACCEs (Fig. 1). We suggest that concurrent pre-existing IHD with an eGFR ≤ 60 mL/min/1.73 m2 (CKD III–V) was a major determinant of digoxin-related MACCEs during the long-term follow-up period.
The MAGIC trial [12] consisted of 57.4% male patients with a CHA2DS2-VASc score of 3, combined β-blocker treatment (40.7% vs. 40.9%) and amiodarone treatment (31.9% vs. 9.9%). Their results were limited by not presenting the maintenance digoxin dose, SDC and renal function status between the digoxin and non-digoxin groups. Digoxin use during acute IHD was not associated with a significant increase in mortality after correcting for clinical characteristics and comorbidities during a short, one-month follow-up.
Until now, the impact of chronic digoxin use in AF patients with pre-existing IHD and renal dysfunction has not been studied. Therefore, our results are noteworthy because we analyze the association between CKD III–V with pre-existing IHD and the occurrence of MACCEs for AF patients taking a chronic digoxin maintenance dose over a long-term follow-up period.
Digoxin increases intracellular calcium concentrations, and the net increase in intracellular calcium activates further calcium release from the sarcoplasmic reticulum, which is responsible for increasing the effect of positive inotropic agents. The negative chronotropic effect of digoxin is largely attributed to increased vagal tone. The optimal SDC is ≤ 0.9 ng/dL with a narrow therapeutic range. A higher SDC could result in dissociated neuro-hormonal effects, a prolonged action potential, myocardial oxygen consumption and arrhythmogenicity, which could be associated with increased mortality [23]. Digoxin is also renally excreted, and doses should be adjusted according to renal function [18,25]. Digoxin toxicity leads to both the frequent occurrence of junctional bradycardia and life-threatening ventricular arrhythmias and may increase cardiac and cerebrovascular ischemic events [11]. Frequent junctional bradycardia in AF may also allow for the development of a cardiogenic thromboembolic source in cerebrovascular events.
Our results suggest that close observation is needed in AF patients with concurrent pre-existing IHD and CKD III–V who are chronically treated with digoxin in order to prevent the occurrence of MACCEs.
Our study has several limitations. First, this study is a retrospective observational analysis in which pre-existing IHD or renal dysfunction was not randomly assigned to AF patients with chronic digoxin treatment. Despite the fact that we had consistent results, the study had a very small population and is likely subject to selection bias with single-center data. Second, we did not take into consideration that frail or elderly patients have poor drug adherence and that OAC therapy might not be the optimal treatment. Third, the data for digoxin use, decreased eGFR, and elevated SDC were derived from hospital admission records for a MACCE, which might be associated with worse outcomes. Fourth, although digoxin is widely used, it is not an essential treatment for AF. Therefore, our results cannot be generalized to the treatment of AF
In conclusion, our data suggest that chronic digoxin treatment in AF patients with concurrent pre-existing IHD and CKD III–V is significantly associated with a greater risk of fatal MACCEs and that these patients should be closely monitored. Large, prospective, multi-center, randomized controlled trials are needed to clarify these results.
Acknowledgments
This study was supported by a grant from the Korean Healthcare Technology R&D project, which is funded by the Ministry of Health & Welfare (2017R1D-1A3B03030919).
Figure 1
Kaplan-Meier curve
It shows major adverse cardiac and cerebrovascular events (MACCE)-free survival in patients with pre-existing ischemic heart disease (IHD) and renal dysfunction (glomerular filtration rate [GFR] ≤ 60 mL/min/1.73 m2, chronic kidney disease stage III–V).
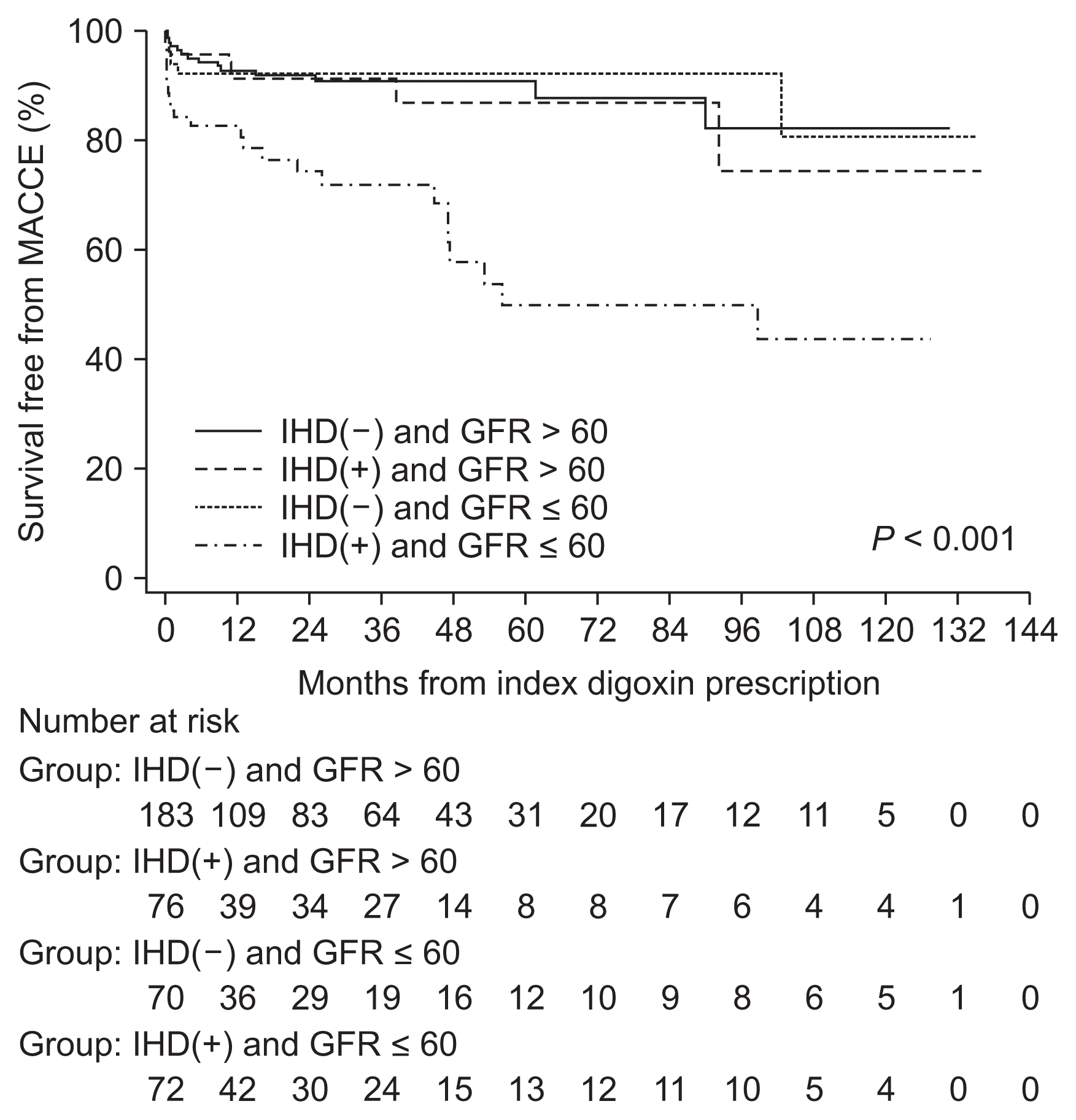
Table 1
Baseline characteristics of patients without and with MACCE
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CKD, chronic kidney disease; eGFR, estimated eglomerular filtration rate; ER, emergency room; ICU, intensive care unit; IHD, ischemic heart disease; JB, junctional bradycardia; MACCE, major adverse cardiac and cerebrovascular events; PAF, paroxysmal atrial fibrillation; TIA, transient ischemic attack.
Table 2
Baseline laboratory characteristics of patients without and with MACCE
Table 3
Cox regression analysis for predictors of MACCE
References
1. Kanji S, MacLean RD. Cardiac glycoside toxicity: more than 200 years and counting. Crit Care Clin 28:527–535. 2012;

2. Van Gelder IC, Rienstra M, Crijns HJ, Olshansky B. Rate control in atrial fibrillation. Lancet 388:818–828. 2016;


3. Ambrosy AP, Butler J, Ahmed A, et al. The use of digoxin in patients with worsening chronic heart failure: reconsidering an old drug to reduce hospital admissions. J Am Coll Cardiol 63:1823–1832. 2014;

4. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64:e1–e76. 2014;

5. Whitbeck MG, Charnigo RJ, Khairy P, et al. Increased mortality among patients taking digoxin--analysis from the AFFIRM study. Eur Heart J 34:1481–1488. 2013;


6. Gheorghiade M, Fonarow GC, van Veldhuisen DJ, et al. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur Heart J 34:1489–1497. 2013;



7. Turakhia MP, Santangeli P, Winkelmayer WC, et al. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol 64:660–668. 2014;



8. Freeman JV, Reynolds K, Fang M, et al. Digoxin and risk of death in adults with atrial fibrillation: the ATRIA-CVRN study. Circ Arrhythm Electrophysiol 8:49–58. 2015;


9. Vamos M, Erath JW, Hohnloser SH. Digoxin-associated mortality: a systematic review and meta-analysis of the literature. Eur Heart J 36:1831–1838. 2015;


10. Chao TF, Liu CJ, Chen SJ, et al. Does digoxin increase the risk of ischemic stroke and mortality in atrial fibrillation? A nationwide population-based cohort study. Can J Cardiol 30:1190–1195. 2014;


11. Spargias KS, Hall AS, Ball SG. Safety concerns about digoxin after acute myocardial infarction. Lancet 354:391–392. 1999;


12. Metawee M, Charnigo R, Morales G, et al. Digoxin and short term mortality after acute STEMI: Results from the MAGIC trial. Int J Cardiol 218:176–180. 2016;


13. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. 2009;



14. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39(2 Suppl 1):S1–S266. 2002;

15. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 60:e44–e164. 2012;

16. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62:e147–e239. 2013;

17. Andrassy KM. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int 84:622–623. 2013;

18. Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 156:570–581. 2012;


20. Koup JR, Jusko WJ, Elwood CM, Kohli RK. Digoxin pharmacokinetics: role of renal failure in dosage regimen design. Clin Pharmacol Ther 18:9–21. 1975;


21. Pastori D, Farcomeni A, Bucci T, et al. Digoxin treatment is associated with increased total and cardiovascular mortality in anticoagulated patients with atrial fibrillation. Int J Cardiol 180:1–5. 2015;


22. Rich MW, McSherry F, Williford WO, Yusuf S. Digitalis Investigation Group. Effect of age on mortality, hospitalizations and response to digoxin in patients with heart failure: the DIG study. J Am Coll Cardiol 38:806–813. 2001;


23. Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA 289:871–878. 2003;


24. Washam JB, Stevens SR, Lokhnygina Y, et al. Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: a retrospective analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Lancet 385:2363–2370. 2015;


- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print
















