Glomerulonephritis following COVID-19 infection or vaccination: a multicenter study in South Korea
Article information
Abstract
Background
Despite the widespread impact of the severe acute respiratory syndrome coronavirus 2 (coronavirus disease 2019, COVID-19) and vaccination in South Korea, our understanding of kidney diseases following these events remains limited. We aimed to address this gap by investigating the characteristics of glomerular diseases following the COVID-19 infection and vaccination in South Korea.
Methods
Data from multiple centers were used to identify de novo glomerulonephritis (GN) cases with suspected onset following COVID-19 infection or vaccination. Retrospective surveys were used to determine the COVID-19–related histories of patients who were initially not implicated. Bayesian structural time series and autoregressive integrated moving average models were used to determine causality.
Results
Glomerular diseases occurred shortly after the infection or vaccination. The most prevalent postinfection GN was podocytopathy (42.9%), comprising primary focal segmental glomerulosclerosis and minimal change disease, whereas postvaccination GN mainly included immunoglobulin A nephropathy (IgAN; 57.9%) and Henoch-Schönlein purpura nephritis (HSP; 15.8%). No patient progressed to end-stage kidney disease. Among the patients who were initially not implicated, nine patients with IgAN/HSP were recently vaccinated against COVID-19. The proportion of glomerular diseases changed during the pandemic in South Korea, with an increase in acute interstitial nephritis and a decrease in pauci-immune crescentic GN.
Conclusion
This study showed the characteristics of GNs following COVID-19 infection or vaccination in South Korea. Understanding these associations is crucial for developing effective patient management and vaccination strategies. Further investigation is required to fully comprehend COVID-19’s impact on GN.
Introduction
South Korea was among the early-hit countries during the novel coronavirus severe acute respiratory syndrome coronavirus 2 (coronavirus disease 2019, COVID-19) pandemic, with the first confirmed case reported on January 20, 2020. By December 2022, over 29 million cumulative COVID-19 cases were diagnosed, accounting for 56.3% of the total population, while COVID-19 vaccination doses exceeded 94 million in South Korea [1,2]. Both COVID-19 infection and vaccines have been linked to autoimmune glomerulonephritis (GN) through the activation of innate and/or adaptive immune responses [3,4]. Additionally, COVID-19 is associated with acute kidney injury (AKI) due to excessive cytokine levels and endothelial damage, which are common extrapulmonary complications [3]. The incidence and characteristics of kidney disease following COVID-19 infection or vaccination in South Korea remain unclear, with sporadic reports from single institutions regarding temporal relationships with COVID-19 vaccination [5–8].
Published cases have indicated that COVID-19 and vaccination are predisposing factors for kidney disease [3,4,9–14]. Following COVID-19, AKI has emerged as a common complication, and certain glomerular diseases have also been associated with COVID-19 [3,13,14]. A population-based retrospective study also revealed an increased risk of glomerular disease relapse after receiving COVID-19 vaccines [15]. These findings underscore the importance of recognizing COVID-19 when managing patients with kidney disease. To effectively respond to the ongoing pandemic and future developments in COVID-19 [16], understanding how patients with GNs are affected by COVID-19 events in real-world settings is crucial.
This study comprehensively characterizes kidney diseases following COVID-19 infection or vaccination in South Korea, utilizing data from multiple hospitals. We also investigate the potential impact of COVID-19 on the occurrence of kidney diseases during the pandemic era.
Methods
The study protocol was approved by the Institutional Review Board (IRB) of Yonsei University Health System (No. 4-2022-1236). Written consent to publish was waived by the IRB due to the retrospective nature and minimal expected risk in this study.
Study design
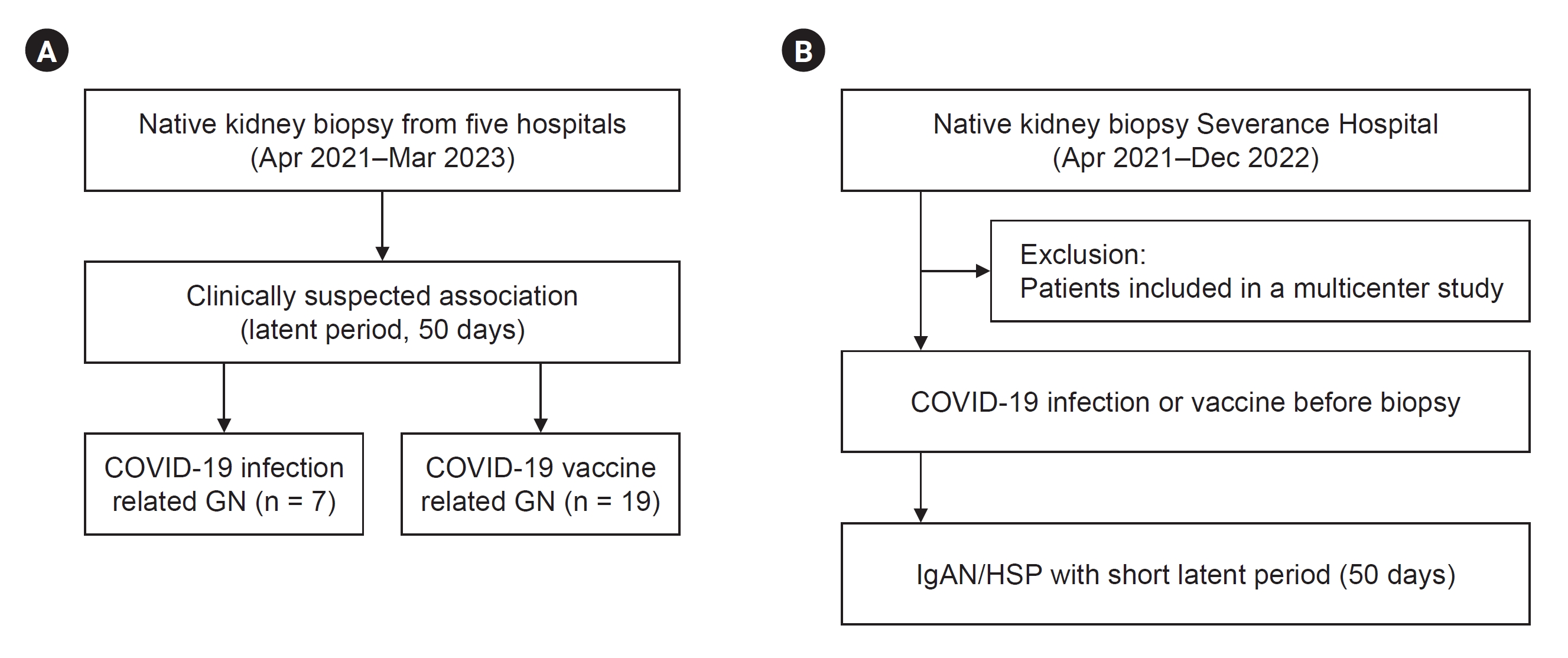
This study addresses three main issues. We identified all de novo GN cases with suspected onset after COVID-19 infection or vaccination from multiple centers (Fig. 1A). Second, we determined the COVID-19–related histories among patients who were initially not implicated by identifying close temporal relationships in a single center (Fig. 1B). Finally, we examined the changes in biopsies during the COVID-19 vaccination or pandemic era. The histopathological review included the use of light microscopy with periodic acid-Schiff, trichrome, silver, and hematoxylin and eosin staining, immunofluorescence microscopy for immunoglobulins and complements, and electron microscopy. The biopsies for the transplanted kidney were excluded. The detailed methods are provided below.
De novo glomerulonephritis cases after COVID-19 infection or vaccination: multicenter analysis
Data were retrospectively collected from multiple centers (Severance Hospital, Yongin Severance Hospital, Wonju Severance Christian Hospital, Ilsan Paik Hospital, and Busan Paik Hospital) between April 2021 and March 2023 using a de-identified electronic documents (Fig. 1A). Patients with a clinically suspected association between COVID-19 events and GNs were included. The clinical onset of GNs in relation to COVID-19 events was evident in collected patients, most of whom had latent period of no more than 50 days after being infected or vaccinated, although the exact latent period remains unknown in some patients. Renal pathology experts thoroughly reviewed all biopsies, and relevant laboratory findings were extracted from electronic medical records. Notably, two cases demonstrating temporal associations with COVID-19 vaccines, lupus nephritis and pauci-immune crescentic GN, have been reported previously [8,17].
Investigation of COVID-19–related history in patients with biopsy-proven kidney disease in a single center
This study focused on elucidating the history of COVID-19 vaccination and infection in patients with GN. We identified COVID-19–related histories among patients who were initially not suspected of having an association with COVID-19 events. To assemble this subset of previously unsuspected biopsies, we excluded those analyzed in the multicenter series and those taken before April 2021 when the nationwide vaccination program commenced. Therefore, we meticulously collected vaccine type, vaccination date, and infection date data from patients who underwent native kidney biopsies at Severance Hospital between April 2021 and December 2022 (Fig. 1B). The investigation was conducted during outpatient visits, and comprehensive information was gathered by reviewing electronic medical records.
Concerning patients with included immunoglobulin A nephropathy (IgAN) or Henoch-Schönlein purpura nephritis (HSP), we conducted an additional investigation into the time of onset. Utilizing the same timeframe (50 days) as the multicenter case series, we identified IgAN and HSP cases that could be temporally linked to vaccination.
Changes in glomerulonephritis diagnoses during the COVID-19 pandemic
We analyzed the changes in GN diagnoses throughout the prepandemic and pandemic periods (2016–2022) at Severance Hospital. For statistical purposes, the biopsies were broadly categorized into 10 groups, including IgAN/HSP, podocytopathy (comprising minimal change disease [MCD] and focal segmental glomerulosclerosis [FSGS]), immune complex-mediated GN (encompassing lupus nephritis, C3 GN, and membranoproliferative GN), membranous nephropathy, other nephropathies (e.g., diabetes mellitus nephropathy, hypertensive nephropathy, or paraprotein-related renal disease), pauci-immune crescentic GN (associated with anti-neutrophil cytoplasm antibodies), acute interstitial nephritis (AIN), acute tubular injury (ATI), thrombotic microangiopathy (TMA), or nonspecific histology. Secondary FSGS cases attributed to clear provocative lesions (e.g., hypertensive nephropathy) were classified accordingly. Biopsies with insufficient glomeruli for a definitive diagnosis were excluded, and the nonspecific histology category was applied to cases with near-normal glomeruli, tubulointerstitium, and blood vessels.
Statistical analyses
Continuous variables are expressed as means with standard deviations (SD) for normally distributed data and medians with interquartile ranges (IQR) for non-normally distributed data, whereas categorical variables are expressed as numbers and proportions. To evaluate the causal effects of COVID-19 vaccination and infection on de novo GNs, a Bayesian structural time-series model was performed using R ‘CausalImpact’ package [18]. The causal effect was determined by assessing the disparity between predicted and observed outcomes. This evaluation helped establish the difference in the proportion of newly diagnosed GN cases at Severance Hospital before and after the commencement of the nationwide vaccination program (April 1, 2021), and before and after the peak of the COVID-19 pandemic (February 1, 2022) in South Korea. This approach considered that the nationwide vaccine program began in March 2021, and COVID-19 cases surged significantly as of February 2022 in South Korea, considering weeks of latent period [1,2,4,14]. An additional time-series analysis was conducted using an autoregressive integrated moving average (ARIMA) model. The Box-Cox transformation was performed for non-normally distributed time-series data. The auto.arima function in R ‘forecast’ package was used to automatically select an optimal model [19]. The Ljung-Box Q statistic was used to evaluate the goodness of fit of the automatically selected ARIMA models. The p-value for Ljung-Box Q statistics > 0.05 indicates that the selected ARIMA model fits well and no autocorrelation exists in the residual. Both step and slope changes following the interventions (i.e., COVID-19 vaccination or infection) were examined. All statistical analyses were performed using R (version 4.2.3; R Foundation for Statistical Computing, http://www.r-project.org) with a significance level of p < 0.05.
Results
Clinical characteristics of patients with glomerulonephritis cases following COVID-19 infection
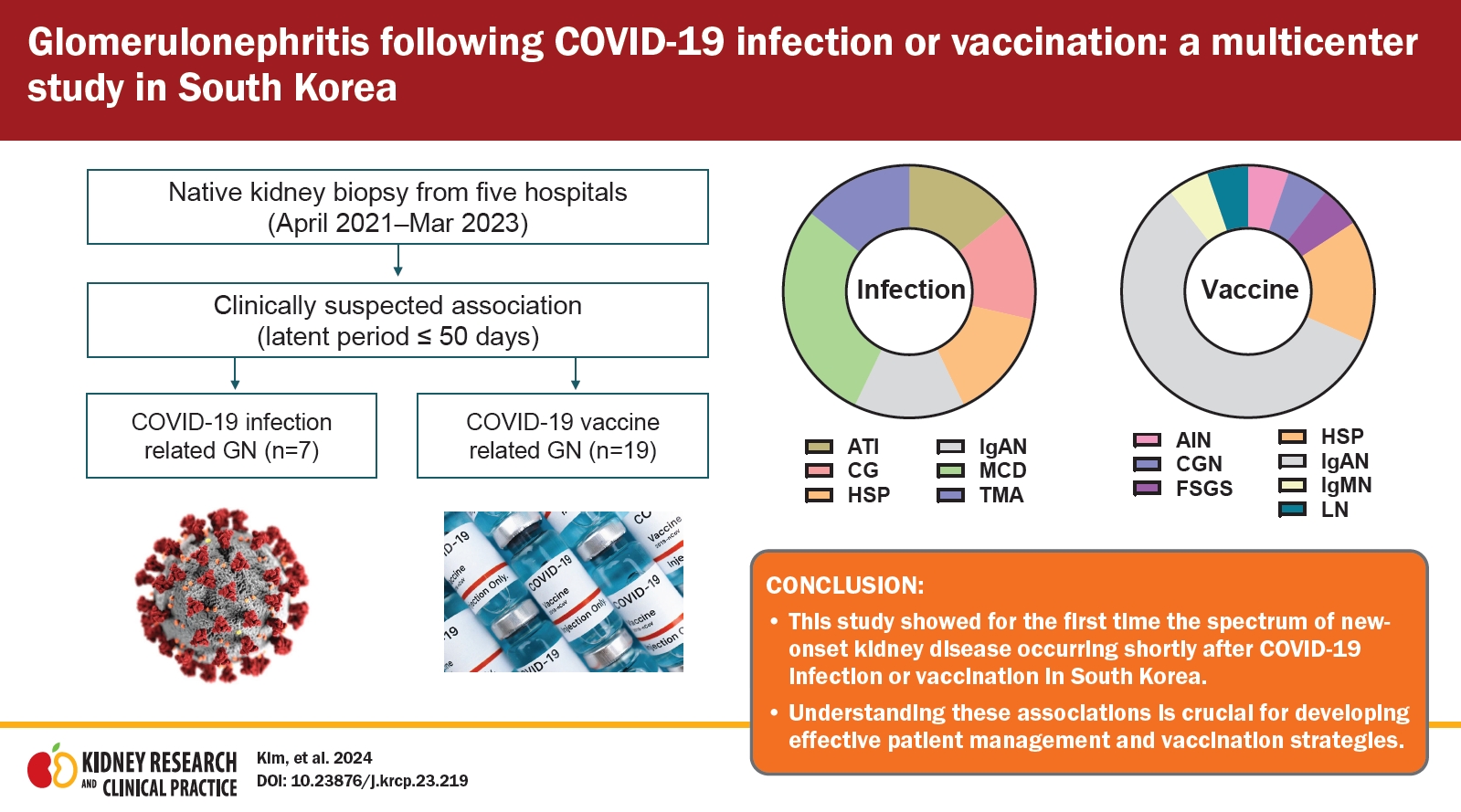
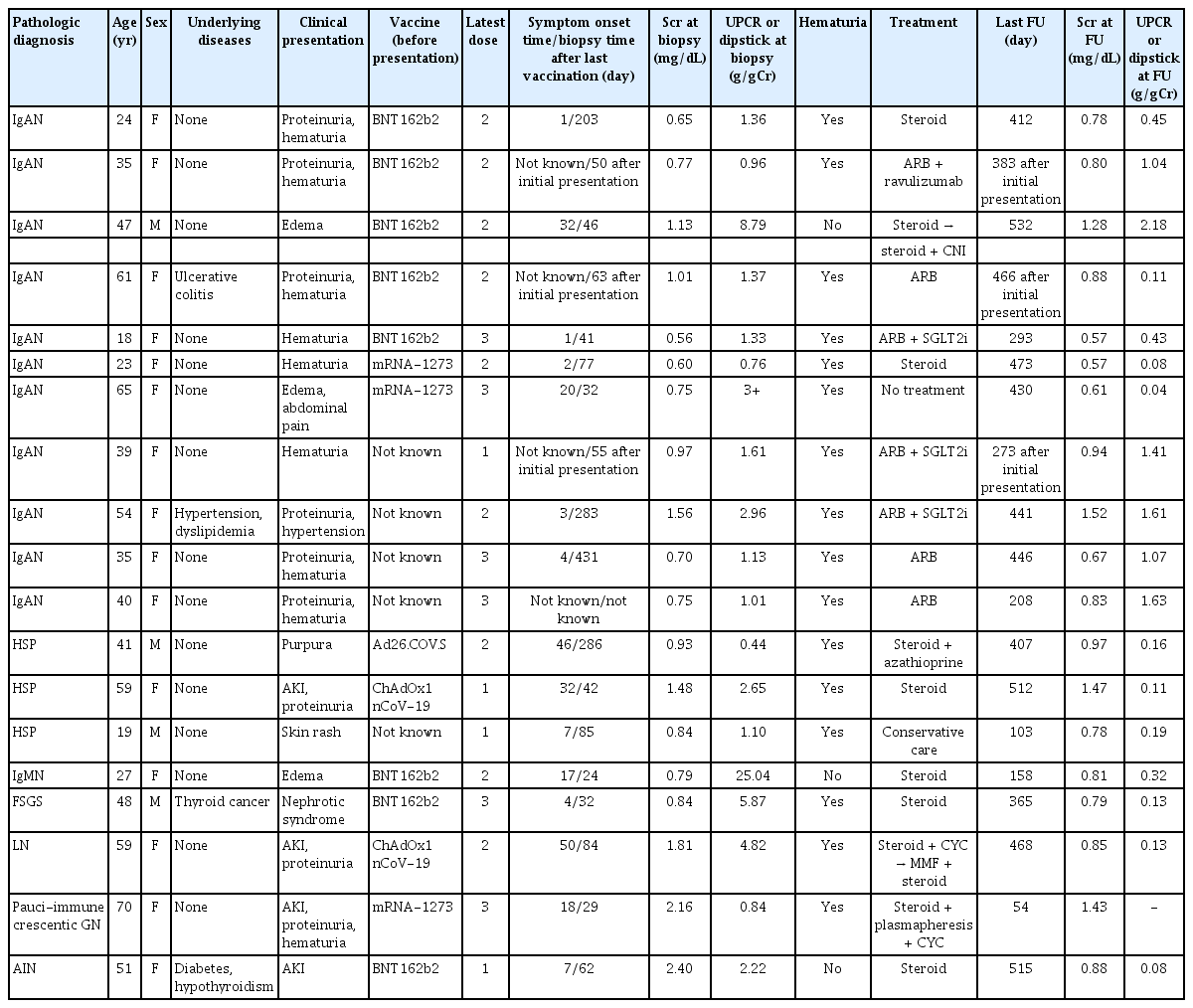
Clinical information from seven patients whose GN was suspected of developing following COVID-19 was collected in a multicenter study. The age at renal biopsy ranged from 24 to 76 years. The pathological diagnoses included ATI (14.3%), TMA with hypertensive nephropathy (14.3%), FSGS, collapsing variant (14.3%), MCD (28.6%), IgAN (14.3%), and HSP (14.3%). Patients with FSGS and IgAN had concurrent ATI. AKI with or without hematuria and proteinuria was the predominant clinical manifestation in three patients (42.9%). These symptoms were observed between 10 and 35 days after infection (Table 1).
Clinical characteristics of patients with glomerulonephritis cases following COVID-19 vaccination
Nineteen patients in whom GN was suspected after COVID-19 vaccination were recruited from multiple centers. The collected GNs were composed of IgAN (n = 11, 57.9%), HSP (n = 3, 15.8%), FSGS (n = 1, 5.3%), , immunoglobulin M nephropathy (n = 1, 5.3%), lupus nephritis (n = 1, 5.3%), pauci-immune crescentic GN (n = 1, 5.3%), and AIN (n = 1, 5.3%). The age at the time of renal biopsy ranged from 18 to 70 years, and the main symptoms were proteinuria, hematuria, edema, and AKI. Both messenger RNA (mRNA)-based vaccines (BNT162b2, n = 8; mRNA-1273, n = 3) and adenovirus-based vaccines (ChAdOx1 nCoV-19, n = 2; Ad26.COV.S, n = 1) were administered. Except for one patient with HSP who developed 32 days after the first dose of ChAdOx1 nCoV-19 and another patient with AIN who developed 7 days after the first dose of BNT162b2, the initial symptoms developed after the second or third dose. The time interval between the first vaccination and symptoms varied from 1 to 50 days. Seven patients experienced symptoms within a short time, even within 1 week after vaccination, while others had a more extended interval. Some patients underwent kidney biopsy more than a year after symptom onset, whereas those who showed acute symptoms underwent kidney biopsy within 1 to 2 months. Most patients received steroids or other immunosuppressive therapies. However, progression to end-stage kidney disease (ESKD) was not observed among the patients during follow-up (Table 2).
Association with COVID-19 or vaccination in unsuspected kidney biopsies
From April 2021 to December 2022 at Severance Hospital, 432 patients underwent native kidney biopsies, and 12 patients (2.8%) were included in the multicenter series of GNs following COVID-19 or vaccination. Among the remaining 420 patients whose connection to COVID-19–related events was initially unknown, a survey was successfully conducted to investigate COVID-19 infection or vaccination history in 143 patients.
Among 143 patients, 29 (20.3%) had preceding COVID-19 infection. Renal biopsy was conducted on an average of 99.0 days (SD, 62.1 days) after infection, and the most common histological diagnosis was IgAN/HSP. Twenty-seven patients (93.1%) also received vaccinations before tissue examination. The most commonly administered vaccine was BNT162b2. The average interval between vaccination and kidney biopsy was 347 days (Supplementary Table 1, available online).
Eighty-three patients (58.0%, 83 of 143) without a history of COVID-19 received vaccination before biopsy. The BNT162b2 vaccine was the most commonly used vaccine. The median days from the first vaccination to the kidney biopsy was 140.0 (IRQ, 77.0–272.0) (Supplementary Table 2, available online). In contrast, 24 individuals (16.8%, 24 of 143) had no history of COVID-19 infection or vaccination before biopsy. There was no significant difference in demographics or pathological diagnoses between the groups with or without a vaccination history, and IgAN/HSP was the most common diagnosis in both groups (Supplementary Table 2, available online).
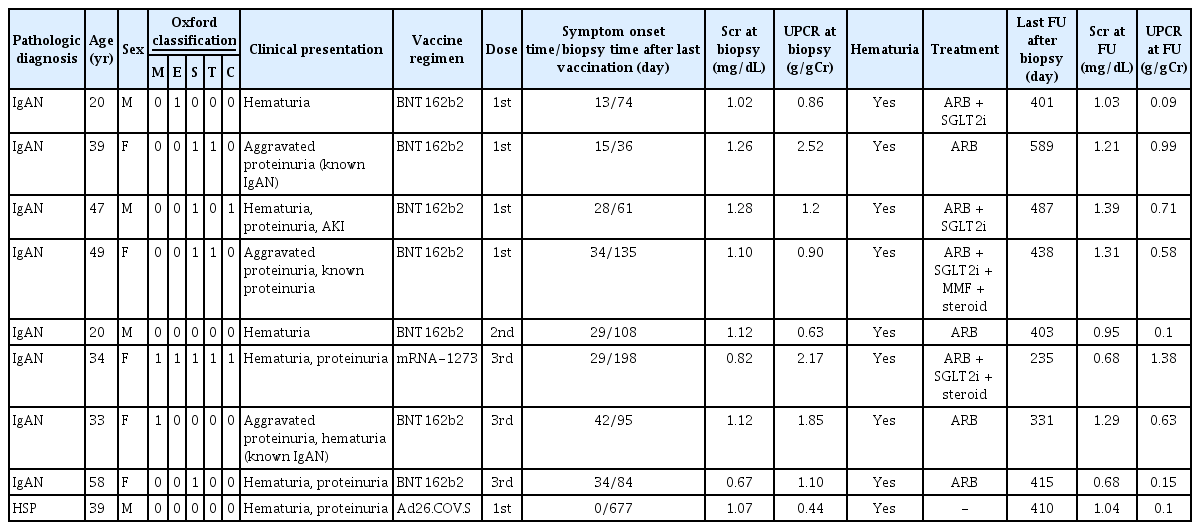
Because IgAN and HSP were the leading causes of postvaccination GNs in multiple centers, we further focused on postvaccination IgAN/HSP (latent period, <50 days) among the patients with a prior vaccination history by analyzing the duration to symptomatic onset. Nine patients with IgAN/HSP (two patients with a history of IgAN) who developed symptoms within 50 days postvaccination and did not have any history of COVID-19 infection were selected. Seven (77.8%) were vaccinated with BNT162b2, and the other two (22.2%) were vaccinated with Ad26.COV.S. Five patients (55.6%) had their first vaccination before onset, whereas one (11.1%) and two (22.2%) had the second or third doses, respectively. The symptoms typically include hematuria and proteinuria. One patient presented AKI. Two patients received immunosuppressive treatment, and the remaining patients received conservative care for proteinuria. None of the patients developed ESKD (Table 3).
Biopsy diagnosis of glomerulonephritis cases during the COVID-19 pandemic era in South Korea
To examine the changes in GN diagnoses during the COVID-19 pandemic, Bayesian structural time-series models and ARIMA models were applied to analyze patients with kidney biopsies from a single institution. There was no significant difference in the frequency of kidney biopsies before and after the initiation of the nationwide vaccine program or the peak of the COVID-19 pandemic (Supplementary Fig. 1, available online). Bayesian structural time-series models showed that compared with the pre-nationwide vaccine program period, the monthly proportion of AIN, nonspecific histology, podocytopathy, and pauci-immune GN were changed as follows: the proportion of AIN, nonspecific histology and podocytopathy increased by 0.96 (95% confidence interval [CI], –0.11 to 2.00), 2.51 (95% CI, 0.37–4.60), and 5.12 (95% CI, 0.80–9.30), respectively. In contrast, the proportion of pauci-immune GN decreased by –3.71 (95% CI, –0.64 to –1.00) (Fig. 2). Based on the analysis, we found a posterior probability of a causal effect ≥ 95%, indicating a strong causal relationship in the AIN, nonspecific histology, podocytopathy, and pauci-immune GN categories (Supplementary Table 3, available online). Other diseases showed no differences in diagnosis rates before and after the nationwide vaccination program (posterior probability, <95%) (Supplementary Table 3, available online; Fig. 2). Supplementary Fig. 2 (available online) and Supplementary Table 4 (available online) present the predictions of the ARIMA model. Other nephropathy and podocytopathy entities showed significant step changes in the diagnostic rates: –1.627 (95% CI, –2.904 to –0.349) and 0.827 (95% CI, 0.168–1.485), respectively. AIN showed a significant slope change in the diagnosis rate (0.049 [95% CI, 0.013–0.084]). However, no disease was significant for either step or slope changes.
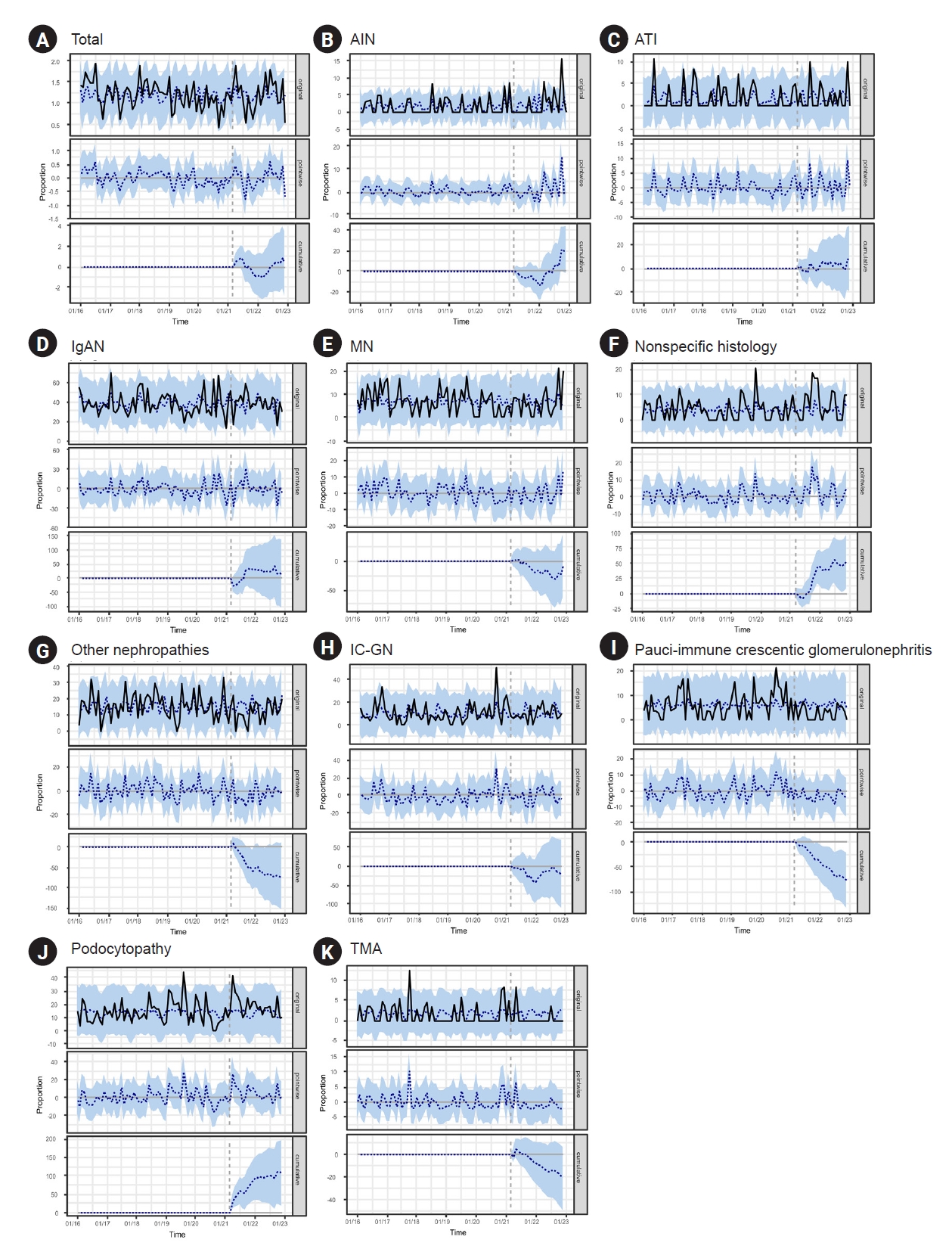
The monthly trend of glomerulonephritis after the initiation of nationwide vaccine program (after April 2021) in Severance Hospital based on the Bayesian structural time-series models.
(A) Total, (B) acute tubular injury (AIN), (C) acute interstitial nephritis (ATI), (D) immunoglobulin A nephropathy (IgAN), (E) membranous nephropathy (MN), (F) nonspecific histology, (G) other nephropathies, (H) immune complex-mediated glomerulonephritis (IC-GN), (I) pauci-immune crescentic glomerulonephritis, (J) podocytopathy, and (K) thrombotic microangiopathy (TMA). The solid lines denote the observed value, the blue-dotted lines denote the predicted value, and the blue shades denote 95% confidence intervals of the predicted value.
Compared with the prepandemic period, only the monthly proportion of AIN was significantly changed in a Bayesian structural time-series model (absolute effect, 3.09 [95% CI, 1.70 to 4.50]; posterior probability of a causal effect, ≥95%) (Supplementary Table 5 and Supplementary Fig. 3; available online). In the ARIMA model, no disease showed a significant difference in either step change or slope change in diagnosis rates (Supplementary Table 6, Supplementary Fig. 4; available online).
Discussion
In this study, which included patients from multiple centers in South Korea, we showed for the first time the spectrum of new-onset kidney disease occurring shortly after COVID-19 infection or vaccination in South Korea. We also determined whether the incidence of GN types changed during the COVID-19 pandemic. Understanding the prevalence and characteristics of COVID-19–related renal disease is critical for monitoring the safety and efficacy of vaccination. More importantly, the results of our study provide insights into the impact of COVID-19–related events on the management of patients with renal disease.
Renal dysfunction is a frequent complication that affects 5% to 37% of hospitalized COVID-19 patients [3]. COVID-19-induced AKI and glomerular disease pathogenesis involve various mechanisms, including hypoperfusion, cytokine storm, endothelial injury, and potential direct kidney infection [3]. In our case series, COVID-19-infected patients displayed diverse glomerular diseases and frequently presented with AKI, likely attributable to COVID-19 [14]. The most frequently observed glomerular disorder was podocytopathy, comprising one case of collapsing variant FSGS and two cases of MCD. The FSGS case exhibited diffuse and severe effacement of the podocyte foot processes, indicating primary FSGS. Collapsing variant FSGS (collapsing glomerulopathy) is the most common type of GN observed in COVID-19 patients. It is believed to be induced by immune dysregulation triggered by COVID-19 [14]. In addition, we identified newly diagnosed IgAN and HSP in COVID-19 patients. It is plausible to assume that COVID-19 upregulates IgA or unmasks subclinical IgAN during the course of the disease, potentially contributing to the development of IgAN and HSP [14,20].
As large-scale vaccination against COVID-19 is underway, concerns about kidney disease that occurs after vaccination have been raised. More than 160 cases of de novo or relapsing GNs after COVID-19 vaccines have been reported [9,21,22]. In our series, the majority of postvaccination GNs (94.7%) manifested as glomerular disease, except for one case of AIN. IgAN (57.9%) and HSP (15.8%) were the most common types of GNs. Among the previously reported GNs that occurred after COVID-19 vaccination [22,23], IgAN was the most common, accounting for 32.6% of the total cases. This trend was also identified in our series where IgAN (57.9%) and HSP (15.8%) were frequent among postvaccination GNs. However, a recent study from Germany reported only one case of IgAN (3.7%) among GNs following a COVID-19–related event [21]. Ethnic differences may be ascribed to the inconsistencies in IgAN occurrence in vaccinated patients [24]. The present study also concurred with previous studies in that postvaccination GNs did not have distinctive features in terms of clinicopathological characteristics [9,25], although the number of patients was too limited to draw a conclusion. The vaccine platforms used against COVID-19 activate diverse immune responses; in particular, mRNA vaccines are known to vigorously enhance immune reactions, which makes it reasonable to consider that COVID-19 vaccines might trigger GNs [4]. While, other reports have challenged this hypothesis. In Switzerland, the incidence of GNs during local vaccination programs was similar to that during the baseline period [25], and a cohort of patients with IgAN did not experience gross hematuria after receiving mRNA-based COVID-19 vaccines [26]. Therefore, the exact risk of developing GNs following COVID-19 vaccination is unclear and is probably very low, emphasizing the benefits of vaccination [10].
In this study, we retrospectively examined the COVID-19 vaccination and infection history of patients who underwent renal biopsy to investigate their relevance to GN. Although it was not possible to survey all patients who underwent renal biopsy, there was no clear evidence of an association between COVID-19 or vaccination history and specific glomerular diseases. Most patients with a history of COVID-19 or vaccination were diagnosed with IgAN. The frequency of IgAN did not appear to change after the pandemic, even when examining the correlation between COVID-19–related history (vaccination or infection) and renal biopsy. However, the worsening of proteinuria after vaccination in patients with preexisting IgAN in this study is consistent with the results of previous studies, suggesting that COVID-19 vaccination may affect IgAN [20,27,28].
The proportion of glomerular diseases increased or decreased significantly after the COVID-19 vaccination program or pandemic. Our Bayesian structural time-series models showed a significant increase in the diagnostic rate of AIN after initiating a nationwide vaccination program or pandemic, supporting the findings of previous case reports [29–31]. AIN after COVID-19 is believed to be a direct effect of a virus-induced immune mechanism or an indirect effect of other conditions associated with COVID-19 [31]. A Bayesian structural time-series model also reported a decrease in the proportion of pauci-immune crescentic GN after the nationwide vaccination program. In contrast, among all kidney biopsies in Germany, no definite increase or decrease in the frequency of pauci-immune crescentic GN was observed during the prepandemic, pandemic, and vaccination periods [21]. Because cases of pauci-immune crescentic GN have been observed after COVID-19 infection or vaccination [32,33], changes in the frequency of pauci-immune crescentic GN should be interpreted with caution. In addition, our results showed that the proportion of patients with nonspecific histology and podocytopathy increased after the nationwide vaccination program. However, because none of the GN showed a significant change in the diagnosis rate in either Bayesian structural time-series models or ARIMA models, it would be difficult to conclude whether the COVID-19 pandemic or nationwide vaccination program had an impact on the incidence of glomerular disease.
This study had some limitations. First, the changes in the diagnosis rate of GN and the investigation of COVID-19–related history among patients undergoing renal biopsy were conducted only at a single center. However, this single-center investigation may have strengths, particularly in terms of ensuring consistency of kidney biopsy and pathologic diagnostic criteria. Second, the pandemic may have affected patients’ utilization of healthcare facilities, leading to potential inaccuracies in the diagnosis rate. However, in South Korea, no strict lockdown fundamentally restricted patients’ access to healthcare facilities. Lastly, COVID-19–related histories obtained through surveys at outpatient clinics can be inaccurate and occasionally rely on patient memory. Nevertheless, there are no available retrospective data from Korea investigating the history of COVID-19 infection or vaccination among patients who have already undergone native renal biopsy.
In summary, this is the first comprehensive report in South Korea characterizing biopsy-proven kidney diseases following COVID-19 or vaccination. This study revealed a spectrum of glomerular and tubulointerstitial diseases, with podocytopathy and IgAN being the leading types postinfection and postvaccination, respectively. The incidence of GNs has changed during the pandemic; however, this requires further investigation. Monitoring and understanding COVID-19–related renal diseases are crucial for effective patient management and vaccination efforts.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.23.219).
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This study was supported by a grant from the Korean Nephrology Research Foundation (Renal Pathology Research Grant 2022). The sponsor had no role in the study design, data collection, or analysis.
Data sharing statement
The data presented in this study are available upon reasonable request from the corresponding author.
Authors’ contributions
Conceptualization: MJ
Data curation, Formal analysis: All authors
Writing–original draft: HWK, MJ
Writing–review & editing: HWK, MJ
All authors read and approved the final manuscript.