A Pragmatic Randomized clinical trial: twice-weekly vs. thrice-weekly Incident hemoDialysis in Elderly patients (PRIDE): study protocol
Article information
Abstract
Background
The optimal frequency for hemodialysis in older adults with end-stage kidney disease (ESKD) has not been established. This study aims to investigate whether a twice-weekly dialysis schedule using an incremental approach can reduce hospitalization rates in the elderly with incident dialysis, compared with conventional thrice-weekly dialysis.
Methods
We have designed a pragmatic randomized controlled trial to compare the effects of twice-weekly versus thrice-weekly hemodialysis in 428 ESKD individuals (dropout rate 20%) aged 60 years or older with residual kidney function (urine output, >500 mL/day). The trial will be conducted across 18 referral hospital-based dialysis centers in Korea. Individual participants will be randomized to either a twice-weekly (with incremental approach) or thrice-weekly dialysis group and will be followed up for 24 months. The primary outcome of the study is all-cause hospitalization rate, while secondary outcomes include dialysis-specific hospitalization rates, mortality, quality of life, frailty, and cost-effectiveness. Participants have the flexibility to transfer to other dialysis centers as needed. The decision to increase dialysis frequency will be made by the treating physicians. The study is ongoing and will be completed in May 2026.
Conclusion
This study will provide valuable insights into the benefits and risks of twice-weekly dialysis with an incremental approach in elderly with residual kidney function compared to thrice-weekly dialysis.
Introduction
End-stage kidney disease (ESKD) poses a substantial public health challenge, with the number of patients requiring renal replacement therapy (RRT) globally reaching 2.61 million in 2010 and projected to rise to 5.43 million by 2030 [1,2]. In Korea, the incidence of hemodialysis (HD) has also been gradually increasing. The increase in HD incidence in South Korea is significantly linked to the aging population. Korea is one of the most rapidly aging countries in the world and the age of dialysis patients is also increasing, with more than half over the age of 60 [2,3].
HD has potential advantages over conservative management or peritoneal dialysis in older adults [3,4]; however, guidelines for optimal HD for the elderly have not yet been established [5]. Typically, patients receive HD three times a week, with only a small proportion of patients receiving less frequent dialysis [6]. However, potential disadvantages such as hemodynamic stress, vascular access problems, bleeding, falls and economic cost should be considered in older adults with ESKD.
In elderly patients, it is often difficult to maintain a thrice-weekly HD schedule due to the presence of other medical conditions and the challenges of frailty. Elderly patients have shown poor outcomes even after initiation of HD. A study by Santos et al. [7] demonstrated a more than two-fold increased risk of mortality at 6 months in patients older than 75 years compared to those younger than 75 years. Another study using Japanese National Dialysis Registry data revealed a 30% mortality rate in those aged over 80 years within 1 year after initiation of HD, with frailty being one of the most important factors associated with early death after initiation of HD [8]. Excessive HD in elderly patients can lead to malnutrition, low blood pressure during dialysis, poor quality of life, depression, and stress due to physical and temporal activity restriction [9]. The increased risk of falls in HD patients is also a serious problem [10].
Incremental initiation of HD involves starting HD at a lower intensity than the standard 4 hours thrice weekly and gradually increasing the frequency and duration of dialysis as kidney function declines [11]. The decision to initiate patients on incremental HD can be made based on clinical parameters such as urine volume or residual kidney function (RKF), socioeconomic factors such as financial limitations or insurance coverage, or lack of availability of dialysis services. Current guidelines recommend that twice-weekly dialysis be performed in patients with kidney urea clearance greater than 3 mL/min/1.73 m2 or a urine output over 0.5 L per day [12,13].
A systemic review and meta-analysis showed no difference in mortality, hospitalization rates, or quality of life between patients receiving incremental and conventional HD, with improved preservation of residual renal function and a reduction in dialysis cost with incremental HD [11]. One randomized controlled trial (RCT) showed no difference in episodes of fluid overload or hyperkalemia, but an increased risk of hyperkalemia with incremental HD [14]. Another study showed a lower hospitalization rate in incremental HD compared to conventional dialysis [15]. These studies demonstrate the need for a large RCT comparing incremental and conventional HD [14,15].
However, current studies do not provide conclusive evidence on the benefits and risks of incremental HD in elderly patients. Therefore, we conducted a pragmatic RCT (Clinical Research Information Service [CRIS], KCT0006853) to determine whether the initiation of RRT with twice-weekly HD reduces hospitalization rates compared to conventional thrice-weekly HD in older adults with ESKD.
Methods
Funding and governance
The trial is sponsored by the National Evidence-based Healthcare Collaborating Agency, Korea (No. HC21C0059; https://www.neca.re.kr/) and the Korean Society of Nephrology (KSN). This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Ethics
This study received ethical approval from the Institutional Review Board (IRB) at each participating clinical center in 2021 to 2022 (see Additional information) and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. The clinical trial has been registered with the CRIS (registration No. KCT0006853).
Study objectives
The overall objective of the ‘Pragmatic Randomized clinical trial: twice-weekly vs. thrice-weekly Incident hemoDialysis in Elderly patients (PRIDE)’ trial is to determine the effects of incremental HD in older adults with RKF. Our specific objectives are as follows:
1) Development of an optimal HD prescription for elderly patients
2) Assessment of the medical benefits and risks of twice-weekly HD
3) Evaluation of the cost-effectiveness of twice-weekly HD
Trial design
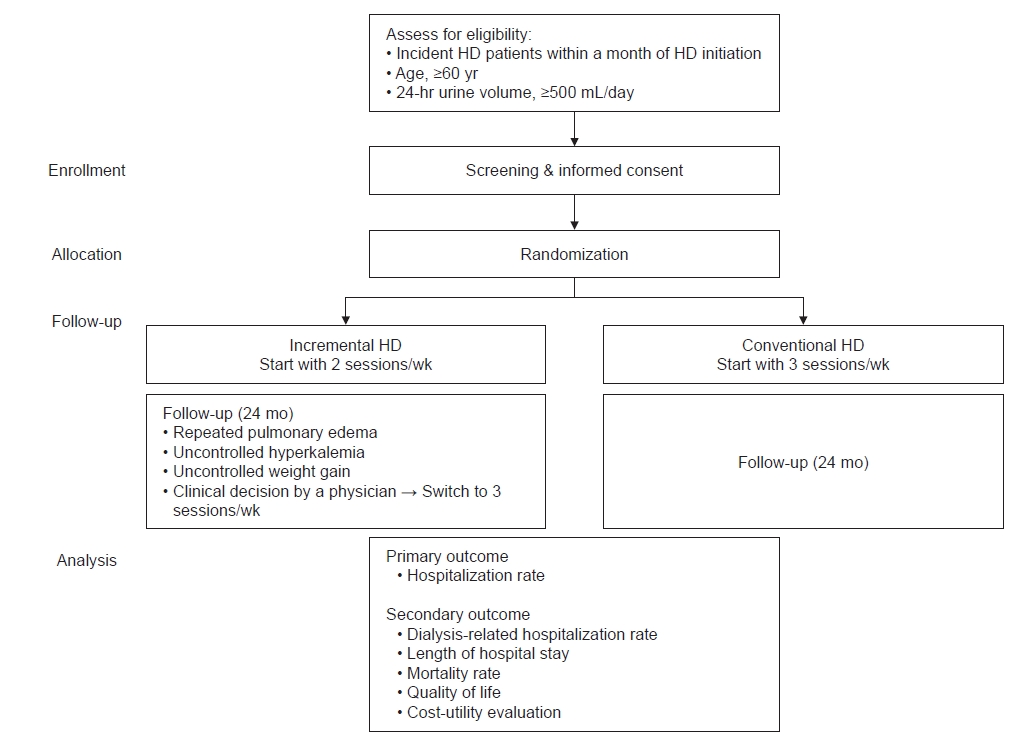
PRIDE trial is designed as a pragmatic RCT comparing the effect of initiating twice-weekly HD with an incremental approach compared to thrice-weekly HD on hospitalization rates in elderly ESKD patients with RKF. A total of 428 participants will be recruited from 18 academic dialysis centers in Korea. Participants will have the flexibility to transfer to other dialysis centers as needed. The decision to increase dialysis frequency will be made by the treating physicians (Fig. 1).
Hypothesis
Our main hypothesis is that twice-weekly HD, when prescribed to older adults with RKF will reduce hospitalization rates compared with thrice-weekly HD.
Inclusion and exclusion criteria
Informed consent is required prior to screening procedures. Inclusion criteria are i) age of ≥60 years; ii) starting maintenance HD within 1 month, iii) RKF defined as a 24-hour urine output of ≥500 mL at randomization, and iv) sufficient understanding of the study procedures and requirements, including the ability to give explicit consent to be randomized. Exclusion criteria are i) presence of heart failure (left ventricular ejection fraction, <40%) or liver cirrhosis, ii) current treatment for an active malignancy or active infection, iii) inability or refusal to provide written informed consent, and iv) enrollment in another clinical trial.
Procedures
Recruitment period
Recruitment began on November 23, 2021 and will continue until the required sample size is reached.
Screening
Patients are prescreened by medical record review. Potentially eligible participants are approached for written informed consent and screened based on the inclusion and exclusion criteria.
Randomization
Screened patients are randomly assigned on a 1:1 basis to a twice or thrice HD protocol. Central randomization is carried out by a randomization list using the random function of SAS version 9.4 (random table; SAS Institute). The randomization is stratified by age (<70 or ≥70 years) and planned/unplanned dialysis. Planned dialysis is defined as the formation of an arteriovenous fistula/arteriovenous graft prior to HD initiation.
Dialysis procedure
The dialyzer, blood flow rate, dialysis duration, and dialysis interval are determined entirely according to the participating center’s protocol and the patient’s situation. Maintenance HD may be performed at the participating center, or patients may be transferred to a local dialysis clinic. The decision to increase session frequency from twice to thrice weekly is made by the treating clinician. Participants are recommended to switch to thrice-weekly HD if they experience uncontrolled pulmonary edema, uncontrolled hyperkalemia, interdialytic weight gain over 3.5 kg, or other medical causes.
Withdrawal from the study
Withdrawal from the study can occur due to kidney transplantation, desire to withdraw consent, or the clinician’s decision.
Data collection and assessment schedule
Table 1 summarizes the data collection and assessment schedule. Study-specific assessment will take place at enrollment and at 3, 6, 12, 18, and 24 months (±4 weeks). At each visit, investigators will review the subjects’ recent medical history, the occurrence of events, frailty, and quality of life. Outside of scheduled visits, the investigators will monitor the participants’ status by telephone.
Outcome measures
The primary outcome is the all-cause hospitalization rate during follow-up. The incidence rates of all-cause hospitalization will be calculated as the total number of hospitalizations per 100 person-years (PYs). If participants have limited mobility and no adequate family or other caregivers to care for them, moving to a nursing home or convalescent hospital is not considered a hospitalization. The secondary outcomes are dialysis-related hospitalization rate, length of hospital stay (hospital days per 1,000 person-days), complications of dialysis, mortality rate and assessments of quality of life, frailty, and cost-utility. For assessment of quality of life, the Kidney Disease Quality of Life Instrument (KDQOL) [16] and the 5-level European Quality of Life 5-dimension version (EQ-5D-5L) [17] are used. The clinical frailty scale [18] and social frailty [19] are used to assess frailty. Willingness to pay is estimated with the questionnaires from previous studies [20–22]. The economic evaluation questionnaires include direct costs, indirect costs, and benefits, which have been modified and reconstructed. Direct costs include out-of-pocket expenses and transportation costs incurred when visiting healthcare providers. Indirect costs include the cost of long-term care insurance for patients, private insurance, and the cost of lost productivity for patients and caregivers.
Adverse and clinical events
Adverse events (AEs) are defined as any death from any cause. The study investigators monitor for the occurrence of AEs throughout the study period. AEs are recorded on an AE case report form. Data on cardiovascular disease-related deaths, infection-related deaths, malignancy-related deaths, musculoskeletal disease-related deaths, deaths related to liver disease, accident-related deaths, death due to other causes, and deaths of unknown causes are collected to assess AEs. The nature of each event and its relationship to dialysis are to be established.
Data is collected for clinical events such as hospitalizations related to dialysis, hospitalizations for reasons other than dialysis, increment of dialysis frequency, procedures or surgeries for vascular access, surgeries for purposes other than vascular access, visits to the emergency room, unscheduled dialysis, visits to an outpatient clinic for new medical issues, changes of residence, changes of dialysis clinic.
Sample size calculations
A previous retrospective study reported that the hospitalization rate among middle-aged (55.2 ± 11.9 years) Korean patients on maintenance HD with frailty was over 50% during 17 months of follow-up [23]. Similarly, the hospitalization rate in the previous prospective observational study was 852 hospitalizations/1,000 PYs in frail patients and 417 hospitalizations/1,000 PYs in non-frail patients (median age, 65 years; interquartile range, 53–73 years) during 1 year of follow-up [24]. Our study population is intended to be older than in previous studies and may be frail. Regarding the hospitalization rates for HD patients were highest in the first year [25], we estimated the hospitalization rate in the conventional dialysis group during the study period (2 years) to be about 60 hospitalizations/100 PYs. A recent prospective study reported a lower hospitalization rate in patients initiating twice-weekly dialysis compared to the conventional HD [15]. Therefore, we estimated a hospitalization rate of 45 hospitalizations/100 PYs in the incremental dialysis group. The sample size for the test group and control group was calculated to obtain at least 80% power at the one-sided significance level of 0.025 (5% on both sides). The dropout rate is assumed to be 20%. Based on this, we calculated (using the R program ‘TrialSize’ package [R Foundation for Statistical Computing]) a sample size of 214 for each group, with the total sample size being 428.
Data collection and management
All participants’ information is recorded by the investigators and clinical research coordinators at the participating dialysis center using electronic case report forms from a web-based database (Korea National Institute of Health; https://icreat.nih.go.kr). The data is handled confidentially. Study participants are only identified by their study identification number. Any personal identifiers are not stored or recorded. An independent management team (academic research office at Soonchunhyang University) is responsible for data management. Through the data validation process, the data is cleaned and the final database is locked. A data monitoring committee board is not needed because this study is a minimal-risk study. To modify the protocol, approval is required following an investigator meeting. Any planned amendments to the trial are communicated to the study site staff in person and reported to the IRB and sponsor. The investigator will also update the protocol in the clinical trial registry. In each participating center, a lead investigator (senior nephrologist) will be identified, to be responsible for identification, recruitment, data collection, and adherence to study protocol and investigator brochure.
Statistical analyses
Statistical analysis will be performed to assess the efficacy and safety variables of the clinical trial. For continuous variables, descriptive statistics will be presented as the number of subjects, mean, standard deviation, median, minimum, and maximum. Categorical variables will be summarized using frequency and percentages. The choice of appropriate statistical methods will depend on the nature of the data (continuous, categorical, or time-to-event data). The methods will include analysis of covariance, chi-square test, Student t test, logistic regression, survival analysis, and repeated measures analysis. Methods such as the chi-square test, Fisher exact tests, or logistic regression will be used to compare the incidence of AEs between treatment groups. Determination of statistical significance will involve calculating a confidence interval or a p-value. A p-value of <0.05 will be considered statistically significant.
We will perform economic evaluations to provide a rigorous health and economic basis for making intervention decisions to optimize HD cycles. To this end, we prospectively collect and analyze extensive cost and multidisciplinary data on intervention implementation and delivery, patient costs, and health-related quality of life from a societal perspective. We intend to use the Markov model, which is the most commonly used research method in economic evaluation studies of interventions for chronic diseases [26]. In addition, to estimate costs at the individual level, patients will be divided into three groups based on their maintenance dialysis schedule: those receiving twice-weekly dialysis, those receiving thrice-weekly dialysis, and those incremented to thrice-weekly from twice-weekly dialysis. The statistical software package SAS version 9.4 or higher will be used to carry out all analyses and to summarize data.
Dissemination plans
The investigators will report the study progress to the sponsor and present at the KSN conference. All results will be published in scientific peer-reviewed journal.
Discussion
The PRIDE study will address the effect on hospitalization rates and safety of twice-weekly HD with an incremental approach vs. thrice-weekly HD in elderly patients. This study will also provide data on quality of life, frailty, and the financial impact of incremental dialysis, not only for patients but also for society. This study design reflects real-world clinical practice.
Our study has several advantages. First, our pragmatic design represents the real-world practice of incremental dialysis. A pragmatic trial often investigates a general approach to treatment rather than dictating the specific details of the approach [27]. In our clinical practice, most patients start dialysis at a referring hospital and transfer to a private clinic. The decision to change dialysis prescription is also made by the treating nephrologist. Therefore, the setting of our study matches with the usual dialysis care setting. The investigators will observe the effect of the initial dialysis prescription. The switch from twice-weekly to thrice-weekly dialysis is a very critical point in this study. The criteria for switching were presented as “uncontrolled pulmonary edema, uncontrolled hyperkalemia, interdialytic weight gain of >3.5 kg and other medical reasons,” leaving room for clinicians to change the number of dialysis if necessary. In the course of the study, we will carefully analyze the reasons for changing the number of dialysis sessions so that these can be presented in detail in the final report.
In addition, this study could provide cost-effectiveness of dialysis frequency. Currently, the elderly dialysis population is increasing worldwide, and the economic burden of kidney disorders is rising [28,29]. Health economic evaluations are now commonly included in pragmatic RCTs that inform policy decisions [30]. The cost-effectiveness analysis will be conducted with QALYs (quality-adjusted life years). In addition, a sensitivity analysis will be conducted to support the results. To estimate costs from the patient’s perspective for the economic evaluations, relying solely on recall through questionnaires during visits limits data accuracy. To address these limitations, we have developed a cost diary to track costs incurred, thereby improving the accuracy of cost data collection related to HD [31].
Furthermore, we will compare the quality of life of patients receiving twice-weekly and thrice-weekly HD. Although dialysis contributes to better survival compared to conservative treatment, dialysis is sometimes used as a part of futile life-sustaining treatment [32]. If less frequent dialysis is associated with better quality of life in older adults, twice-weekly dialysis may be a valid option. We selected the all-cause hospitalization rate as the primary outcome. Hospitalizations are clinically relevant in older adults with ESKD. Hospitalization brings major changes and affects the quality of life. Older adults with ESKD have high hospitalization rates due to frailty, multimorbidity, and dialysis-specific complications such as dialysis access problems [33]. Therefore, hospitalization is a major concern for dialysis patients and can be a significant burden to the family and society.
Aging and frailty often affect disease management in older adults. Multimorbidity, functional status, polypharmacy, decreased drug clearance prolonged drug half-life, and cognitive impairment should be taken into consideration in the management of elderly patients. Older people are the most heterogeneous of any age group in terms of physical, psychological, social, and functional characteristics. Additionally, the need for functional assistance increases with age [34]. As a result, treatment goals and methods are often different for the elderly. For example, the American Diabetes Association recommends that those with multiple coexisting chronic conditions, cognitive impairment, or functional dependence should have less stringent glycemic goals [35]. The 2018 European Society of Cardiology and the European Society of Hypertension (ESC/ESH) guideline also recommends higher blood pressure treatment targets in patients aged over 80 years [36].
Unfortunately, dialysis guidelines for the elderly are scarce, probably due to a lack of evidence. There is still controversy over what the optimal dialysis regimen is for older adults [5,37]. The dialysis needs of older and frail patients may differ from those of younger and fitter patients. Reduced resting energy expenditure and reduced dietary intake in frailty may alter the dialysis requirements. Fluid removal targets for maintenance HD in the elderly may be altered to achieve blood pressure control and minimize long-term cardiac complications, rather than to reduce symptoms and preserve residual renal function. In addition, conventional dialysis in older adults could result in distressing symptoms that do not restore health such as intradialytic hypotension, decline in functional status, post-dialysis fatigue, and significant caregiver burden.
This study was prompted by concerns about the optimal use of HD in older adults. Although dialysis has significant benefits over conservative treatment, HD itself has many side effects. In particular, the risk of falls and hypotension is greater in frail patients, but frailty is not considered in current treatment guidelines when determining the appropriateness of HD. Frailty is an important determinant of patient-specific outcomes in dialysis patients [38,39]. It is expected that this study will provide evidence on the impact of frailty on conventional dialysis in the elderly.
Notes
Additional information
The approval numbers from Institutional Review Boards of all 18 participating sites are as follows: Soonchunhyang University Bucheon Hospital, SCHBC 2021-12-010; Soonchunhyang University Seoul Hospital, SCHUH 2021-07-009; Pusan National University Hospital, 2201-001-110; Pusan National University Yangsan Hospital, 05-2021-300; Wonkwang University Hospital, WKUH 2021-11-028; Korea University Guro Hospital, 2022GR0038; Yonsei University Wonju College of Medicine, CR321163; The Catholic University of Korea, Yeouido St. Mary’s Hospital, SC21OIDC0201; The Catholic University of Korea, Daejeon St. Mary’s Hospital, DC19OEDI0001; Kangbuk Samsung Hospital, KBSMC 2021-12-027; Ulsan University Hospital, UUH 2022-01-008; Gyeongsang National University Changwon Hospital, GNUCH 2021-12-029; Keimyung University Dongsan Hospital, DSMC 2021-12-049; Presbyterian Medical Center, 2022-01-003; Hallym University Chuncheon Sacred Heart Hospital, CHUNCHEON 2021-12-002; Konyang University Hospital, KYUH 2022-01-005; Dongguk University Ilsan Hospital, DUIH 2021-12-016-003; National Medical Center, NMC-2022-07-080.
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This manuscript is supported by a grant of Patient-Centered Clinical Research Coordinating Center (PACEN) by the Ministry of Health & Welfare, Republic of Korea (No. HC21C0059), the Korean Society of Nephrology, and the Soonchunhyang University Research Fund.
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: BCY, SC, SHK
Data analysis, Methodology: HJ, CL, HS, JN
Funding acquisition: SHS, SHK
Writing–original draft: HJ, MH, HS
Writing–review & editing JN, SHK
All authors read and approved the final manuscript.