High water intake induces primary cilium elongation in renal tubular cells
Article information
Abstract
Background
The primary cilium protrudes from the cell surface and functions as a mechanosensor. Recently, we found that water intake restriction shortens the primary cilia of renal tubular cells, and a blockage of the shortening disturbs the ability of the kidneys to concentrate urine. Here, we investigate whether high water intake (HWI) alters primary cilia length, and if so, what is its underlying mechanism and its role on kidney urine production.
Methods
Experimental mice were given free access to normal water (normal water intake) or 3% sucrose-containing water for HWI for 2 days. Some mice were administered with U0126 (10 mg/kg body weight), an inhibitor of MEK kinase, from 2 days before HWI, daily. The primary cilium length and urine amount and osmolality were investigated.
Results
HWI-induced diluted urine production and primary cilium elongation in renal tubular cells. HWI increased the expression of α-tubulin acetyltransferase 1 (αTAT1), leading to the acetylation of α-tubulins, a core protein of the primary cilia. HWI also increased phosphorylated ERK1/2 (p-ERK1/2) and exocyst complex component 5 (Exoc5) expression in the kidneys. U0126 blocked HWI-induced increases in αTAT1, p-ERK1/2, and Exoc5 expression. U0126 inhibited HWI-induced α-tubulin acetylation, primary cilium elongation, urine amount increase, and urine osmolality decrease.
Conclusion
These results show that increased water intake elongates the primary cilia via ERK1/2 activation and that ERK inhibition prevents primary cilium elongation and diluted urine production. These data suggest that the elongation of primary cilium length is associated with the production of diluted urine.
Introduction
The primary cilium is a solitary, immotile cellular organelle and is observed in nearly every mammalian cell. This primary cilium functions as a mechanosensor and chemosensor in cells [1,2]. The length of the primary cilium is altered dynamically by cellular conditions. Recent studies have shown that the abnormal structure and function of the primary cilium are associated with several diseases [3–5]. In the kidneys, the primary cilia protrude from the tubular lumens of the tubular epithelial cells, directly contacting the prourine. Previously, we found that unilateral nephrectomy induces primary cilium elongation in the remaining renal tubular cells, which are exposed to increased prourine flow, and that renal tubular cells suffering from fibrosis and stress contain primary cilia of diverse lengths, compared with normal renal tubular cells [5–7]. Furthermore, we found that water intake restriction by discontinuing water supply in mice causes the shortening of the primary cilia in renal tubular cells and that the inhibition of this shortening impedes the kidney’s ability to concentrate urine [8]. These findings suggest that the alteration of primary cilium length is associated with the urine concentration process in the kidneys and kidney diseases. Despite these interesting findings, the role of primary cilium length alteration on urine production and its underlying mechanisms are largely unknown.
The microtubule is a central core component of the primary cilium, and its assembly and disassembly are associated with the elongation and shortening of the primary cilia, respectively [5,9,10]. The assembly and disassembly of microtubules are controlled by α-tubulins posttranslational modifications, such as acetylation and deacetylation [11]. Recent studies have demonstrated that α-tubulin acetyltransferase 1 (αTAT1), which catalyzes the acetylation of α-tubulins, elongates primary cilia in various ciliated cells [12]. By contrast, histone deacetylase 6 (HDAC6) shortens primary cilia by the deacetylation of α-tubulins [11,13]. Recently, we also reported that water intake restriction activates HDAC6 and shortens the primary cilium length in renal tubular epithelial cells and that the inhibition of water restriction-induced activation of HDAC6 using an HDAC6 inhibitor disrupts the kidney’s ability to concentrate urine [8].
Mitogen-activated protein kinases (MAPKs) regulate various cellular functions, including proliferation, differentiation, and cytoskeleton remodeling. Furthermore, studies have reported that ERK is associated with the expression and cellular localization of aquaporin-2 (AQP-2) in renal tubular cells and urine production [14,15]. Trepiccione et al. [14] reported that MAPK inhibitors prevent lithium-induced basolateral localization and downregulation of AQP-2 in the tubular cells. Previously, we found that ERK inhibition prevents primary cilium elongation in renal tubular cells, together with the inhibition of exocyst complex component 5 (Exoc5, which is also called sec10), which serves as an important regulatory protein in primary ciliogenesis [16]. These suggest that primary ciliogenesis is associated with urine production.
Here, we investigate whether high water intake (HWI) alters primary cilium length of kidney tubular epithelial cells, if so, what its underlying mechanism and its role on urine production are. In this study, we show for the first time that water intake increase induces primary cilium elongation along with increased αTAT1 expression, ERK1/2 activation, and Exoc5 expression and that ERK inhibition blocks HWI-induced primary cilium elongation, disturbing the kidney’s urine-producing ability. These findings indicate that the alteration in primary cilium length is involved in the regulation of body water balance.
Methods
Animals
The study analyzed 10- to 12-week-old C57BL/6 male mice (Koatech). All experiments were approved and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Kyungpook National University (No. KNU-2022-0335). Mice were given free access to normal water (normal water intake, NWI) or 3% sucrose-containing water for HWI for 2 days. Some mice were administered with U0126 (10 mg/kg body weight [BW]; Selleckchem), an inhibitor of MEK kinase, or saline (vehicle) from 2 days before HWI daily. Mice were sacrificed 48 hours after HWI supply without fasting. For biochemical and histological experiments, the kidneys were either frozen in liquid nitrogen or perfusion-fixed with periodate-lysine-paraformaldehyde (4% paraformaldehyde, 75-mM l-lysine, 10-mM sodium periodate; Sigma-Aldrich) immediately following retrieval.
Blood and urine biochemistry
To evaluate plasma glucose level, about 30 µL of blood were obtained from the retro-orbital vein plexus using heparinized glass capillary tubes and then plasma was obtained by centrifugation. Concentrations of glucose in plasma were measured using a Vitros250 (Johnson & Johnson). Urine samples were collected using metabolic cages for 4 hours before the mice were sacrificed. The urine samples were subjected to biochemical analysis. Urine and plasma osmolalities were measured using a cryoscopic osmometer (Osmomat 030-D; Gonotec).
Immunofluorescence staining
Kidney paraffin sections were stained with anti–acetylated-α-tubulin (Cat. No. T745; Sigma-Aldrich), anti–Na/K-ATPase (Cat. No. ab76020; Abcam), anti–AQP-1 (Cat. No. AQP-001; Alomone Laboratories), and anti–AQP-2 (Cat. No. AQP-002; Alomone Laboratories) antibodies. To detect the cell nuclei, DAPI was applied to the sections. Images were captured using a Leica microscope (DM2500). Fixed cells were stained with anti–acetylated-α-tubulin for measurement of primary cilium length.
Measurement of the primary cilium length
In this study, 5 to 10 fields per kidney (n = 5) were randomly captured (400×) using a Leica microscope (DM2500), and the primary cilium length was measured in >100 cells using i-Solution software (IMT i-Solution). Cilium length was measured by tracing the cilium curvilinear line with several straight lines as instructed by the user’s guide for i-Solution. Cilium length was measured blindly by a person unaware of the grouping of the samples.
Western blot analysis
Western blot analyses were performed as described previously [17]. The following antibodies were used: anti-αTAT1 (Cat. No. NBP1-57650; NOVUS), anti-HDAC6 (Cat. No. abs134070; Absin), anti– acetylated-α-tubulin (Cat. No. T7451; Sigma-Aldrich), anti–α-tubulin (Cat. No. T7451; Sigma-Aldrich), anti-phosphorylated ERK1/2 (p-ERK; Cat. No. 9101; Cell Signaling), anti-total ERK (t-ERK; Cat. No. sc271269; Santa Cruz Biotechnology), anti-Exoc5 (Cat. No. 17593-1-AP; Proteintech), anti–AQP-2 (Cat. No. ab3274; Merck Millipore), anti-E-cadherin (Cat. No. 610181; BD Bioscience), anti-GAPDH (Cat. No. NB300-221; NOVUS), and anti–β-actin (Cat. No. A2228; Sigma-Aldrich) antibodies.
Measurement of HDAC6 activity
The HDAC6 activity was measured using an HDAC6 activity assay kit (Biovision Inc.) according to the manufacturer’s instructions.
Membrane and cytoplasmic protein extraction
Membrane and cytoplasmic protein extraction in the kidneys was performed using the ExKine membrane and cytoplasmic protein extraction kit (Abbkine) according to the manufacturer’s instructions. Fractions were confirmed by Western blot analysis using anti-E-cadherin (BD Bioscience) and anti-GAPDH (NOVUS) antibodies, as markers of the cell membrane and cytosol, respectively.
Immunochemical staining
Kidney sections and fixed cells were immunostained as described previously [10]. For immunochemical staining, the sections were stained with anti–AQP-2 (Cat. No. AQP-002; Alomone Laboratories) antibody. Hematoxylin was used for counter-staining. Images were captured using a Leica microscope (DM2500).
Cell culture
Madin-Darby canine kidney (MDCK) cells were cultured in MEM with 5% fetal bovine serum and penicillin/streptomycin (penicillin, 100 IU/mL; streptomycin, 100 µg/mL). For immunofluorescence staining of primary cilia, cells were cultured on glass coverslips. When MDCK cells reached 100% confluence, cells were treated with 10-µM U0126 (a MEK inhibitor; Selleckchem) or vehicle (distilled water). The chosen dosage of U0126 was based on previous studies [18]. Two days after U0126 treatment, cells were harvested using cell lysis buffer or fixed with 4% paraformaldehyde and processed for Western blot analyses or immunofluorescence staining, respectively.
Statistics
All data were analyzed using GraphPad Prism 6 software (GraphPad Software, Inc.). Results are expressed as the mean ± standard error of the mean (SEM). Statistical differences among the groups were assessed using the Student t test for comparison between two groups for Fig. 1, 2 and a two-way analysis of variance with repeated measures followed by Tukey multiple comparisons post hoc test for more than three groups for Fig. 3–6 and Table 1. Differences were considered statistically significant at p < 0.05.
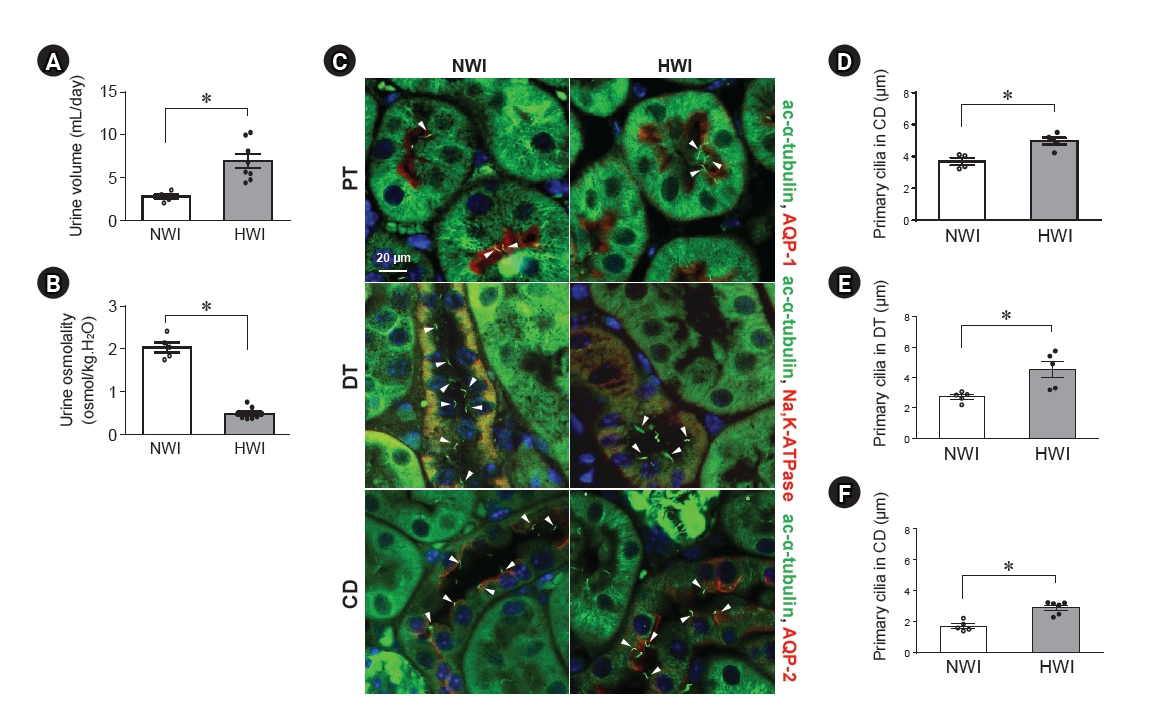
Length of the primary cilia on renal tubular cells after high water intake (HWI).
Mice can freely access normal water (normal water intake, NWI) or 3% sucrose-containing water (HWI) for 2 days. Urine samples were collected using metabolic cages for 4 hours before the mice were sacrificed. Urine volume (A) and osmolality (B) were determined. (C) Kidney sections were costained with anti–acetylated-α-tubulin (ac-α-tubulin, a marker of primary cilia, green), anti–aquaporin-1 (AQP-1; a marker of proximal tubule cells, red), anti–AQP-2 (a marker of principal cells of the collecting duct, red), or anti–Na+-K+-ATPase (a basolateral protein and a marker of distal tubule cells, red) antibodies. DAPI (blue) was used to visualize the nuclei. (D–F) The average values of the primary cilium length were determined in the proximal tubule (PT), distal tubule (DT), and collecting duct (CD) in the cortex. The arrowheads indicate the primary cilia. Results expressed as mean ± standard error of the mean (n = 5–8).
*p < 0.05.
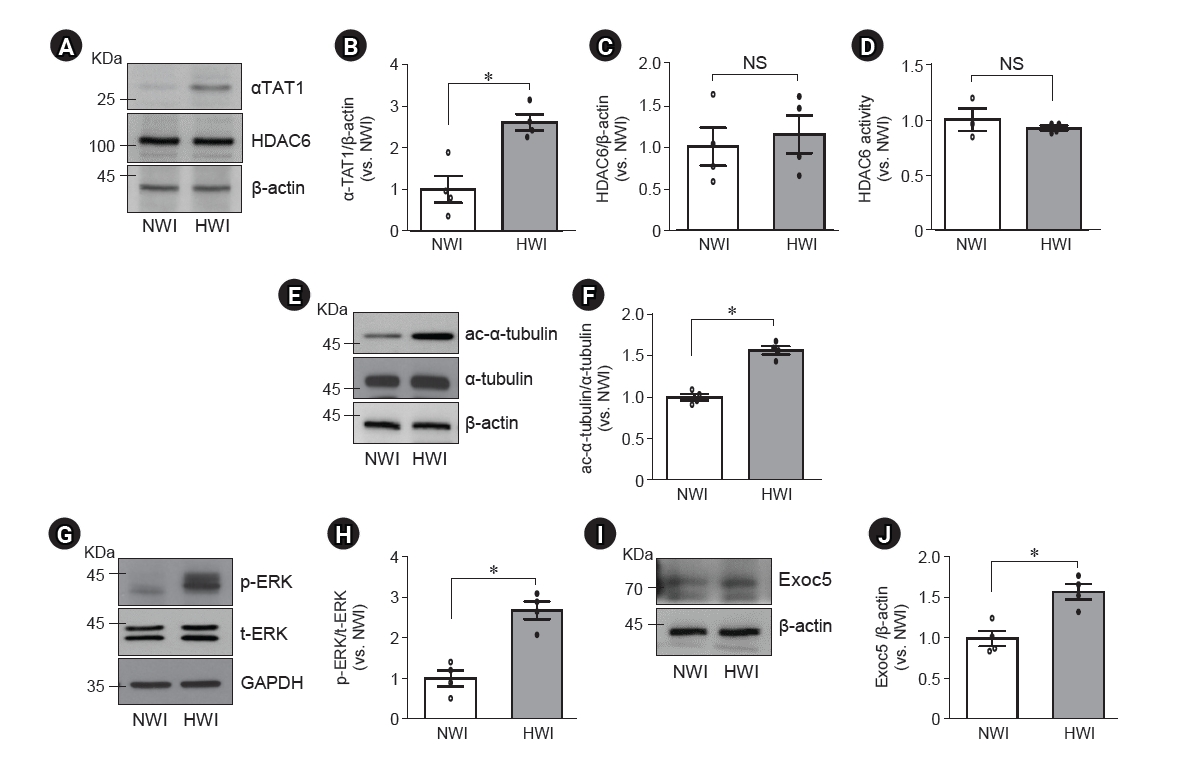
Changes in αTAT1, HDAC6, ac-α-tubulin, p-ERK, and Exoc5 expressions in the kidney after high water intake (HWI).
Mice were given free access to normal water (normal water intake, NWI) or 3% sucrose-containing water (HWI) for 2 days. Kidney samples were subjected to Western blotting (A–C, E–J) or HDAC6 activity (D) assay. Antibodies were anti-αTAT1 (A), anti-HDAC6 (A), anti–ac-α-tubulin (E), anti–α-tubulin (E), anti–p-ERK (G), anti–total-ERK (t-ERK, G), and anti-Exoc5 (I) antibodies. (A, E, G, I) β-actin and GAPDH were used as the loading controls. (B, C, F, H, J) Densities of blots were determined using ImageJ. Results are expressed as mean ± standard error of the mean (n = 4).
ac-α-tubulin, acetylated-α-tubulin; αTAT1, α-tubulin acetyltransferase 1; Exoc5, exocyst complex component 5; HDAC6, histone deacetylase 6; NS, no significant difference; p-ERK, phosphorylated-ERK.
*p < 0.05.
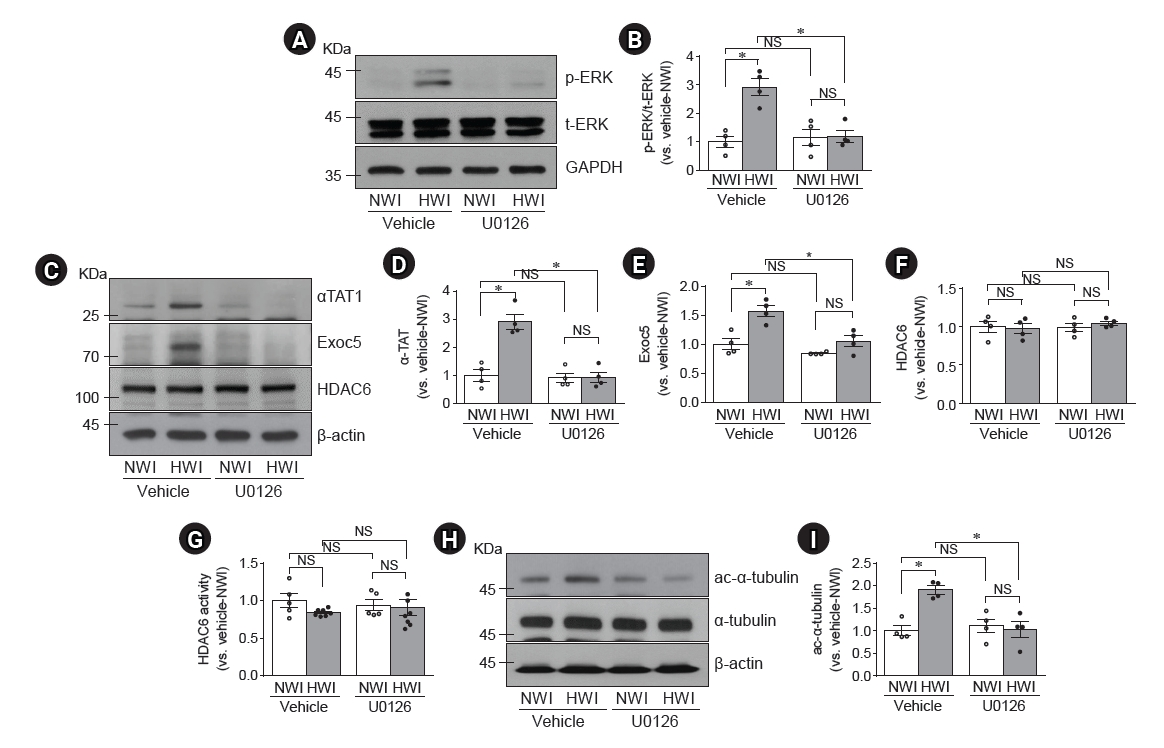
Blockage of high water intake (HWI)-induced p-ERK, αTAT1, Exoc5, ac-α-tubulin expressions and HDAC6 activity by U0126.
Mice were given free access to water (normal water intake, NWI) or HWI with 3% sucrose buffer for 2 days. Some mice were administered either U0126 (10 mg/kg body weight) or saline (vehicle) intraperitoneally daily, starting from 48 hours before HWI until the end of the experiment. (A, C, H) Kidneys were subjected to Western blotting (A–F, H–I) and HDAC6 activity (G) assay. The following antibodies were used; anti–p-ERK (A), anti–total-ERK (t-ERK, A), anti-αTAT1 (C), anti-Exoc5 (C), anti-HDAC6 (C), anti–ac-α-tubulin (H), and anti–α-tubulin (H) antibodies. GAPDH and β-actin were used as the loading control. (G) HDAC6 activity was determined in the whole kidneys. (B, D, E, F, I) Densities of blots were determined using ImageJ. Results are expressed as mean ± standard error of the mean (n = 4–8).
ac-α-tubulin, acetylated-α-tubulin; αTAT1, α-tubulin acetyltransferase 1; Exoc5, exocyst complex component 5; HDAC6, histone deacetylase 6; NS, no significant difference; p-ERK, phosphorylated-ERK.
*p < 0.05.
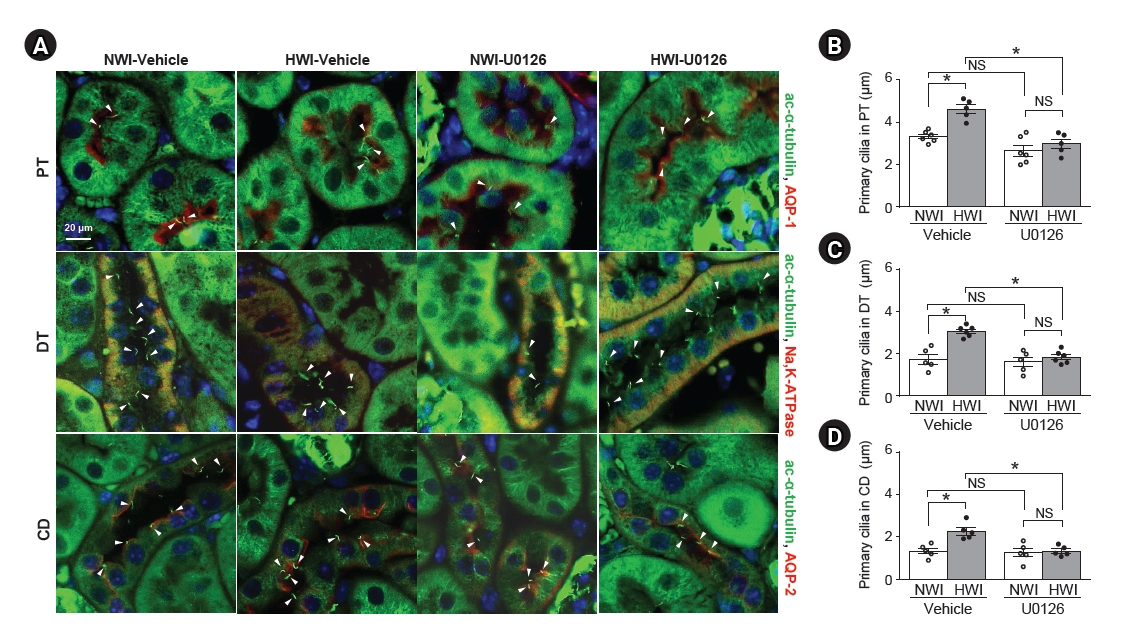
Blockage of high water intake (HWI)-induced primary cilia shortening by U0126 administration.
Mice were given free access to water (normal water intake, NWI) or HWI with 3% sucrose buffer for 2 days. Some mice were administered either U0126 (10 mg/kg body weight) or saline (vehicle) intraperitoneally daily starting from 48 hours before HWI until the end of the experiment. (A) Kidney sections were co-stained with anti–acetylated-α-tubulin (ac-α-tubulin, green), anti–aquaporin-1 (AQP-1, red), and anti–AQP-2 (red), or anti–Na+-K+-ATPase (red) antibodies. DAPI (blue) was used to visualize the nuclei. (B–D) The average values of primary cilium length were determined in the proximal tubule (PT), distal tubule (DT), and collecting duct (CD) in the cortex. Arrowheads indicate primary cilia. Results are expressed as mean ± standard error of the mean (n = 5–6).
NS, no significant difference.
*p < 0.05.

Blockage of primary cilia elongation by U0126 in MDCK cells.
MDCK cells were treated with either 10-µM U0126 or vehicle (saline) for 2 days. (A, B) Cells were fixed and processed for immunofluorescence staining using anti–acetylated-α-tubulin antibody (green). DAPI (blue) indicates nuclear staining. (B) The length of primary cilia was measured. The results are expressed as the mean ± standard erro of the mean (n = 5). (C–F) Cell lysates were subjected to Western blot analysis using anti–exocyst-complex-component-5 (Exoc5), –GAPDH, –phospho-ERK (p-ERK), and –total-ERK (t-ERK) antibodies. (D, F) Band densities were measured using ImageJ software.
MDCK, Madin-Darby canine kidney; NS, no significant difference.
*p < 0.05 vs. vehicle.

Decreased apical expression of AQP-2 after high water intake (HWI) and blockage of this increase by U0126.
Mice were given free access to water (normal water intake, NWI) or HWI with 3% sucrose buffer for 48 hours. U0126 (10 mg/kg body weight) or saline (vehicle) was administered intraperitoneally daily starting from 48 hours before HWI until the end of the experiment. (A, B) Whole-kidney lysates were subjected to Western blotting analysis using anti–AQP-2 antibody. β-actin was used as the loading control. (B) Densities of blots were determined using ImageJ. (C–F) The expression of AQP-2 was assessed in the membrane (C) and cytosolic (E) fractions from the whole kidneys. E-cadherin and GAPDH were used as the loading controls for the membrane and cytosol fractions, respectively. (D, F) Densities of blots were determined using ImageJ. (G) Kidney sections of 3-μm thickness were immunohistochemically stained using anti–AQP-2 antibody (brown). Higher magnification is indicated by the lined rectangles. Results are expressed as means ± standard error of the mean (n = 4).
AQP-2, aquaporin-2; NS, no significant difference.
*p < 0.05.
Results
High water intake elongates primary cilia on renal tubular cells
Mice were given free access to either normal water (NWI) or 3% sucrose-containing normal water (HWI). In this study, a 3% sucrose-containing water significantly increased the amount of water intake in mice compared with NWI (0.53 mL/g BW/day in HWI and 0.28 mL/g BW/day in NWI, p < 0.001). HWI increased the urine output (Fig. 1A). Furthermore, HWI significantly decreased urine osmolality (Fig. 1B). However, plasma glucose concentration (227.3 ± 9.1 in NWI and 249.3 ± 16.5 in HWI, p = 0.15), BWs (22.2 ± 0.4 g in NWI and 22.3 ± 0.3 g in HWI, p = 0.42), and food intake (3.0 ± 0.17 g in NWI and 2.9 ± 0.12 g in HWI, p = 0.19) did not differ between the groups. Also, all mice survived the entire experimental period.
When primary cilia were visualized by immunofluorescence staining using acetylated α-tubulin (a marker of the primary cilia), primary cilia were observed in the lumen of tubules and in most of the tubular epithelial cells in both HWI and NWI groups, except in intercalated cells (Fig. 1C). Among tubular epithelial cells, the primary cilium length in proximal tubular cells was the longest (Fig. 1C). HWI-induced primary cilium elongation in tubular epithelial cells (Fig. 1C–1F).
High water intake increases αTAT1, p-ERK, and Exoc5 expressions but not HDAC6 expression and activity in the kidneys
The elongation and shortening of primacy cilia are regulated by the assembly and disassembly of the microtubules through the acetylation and deacetylation of α-tubulins, respectively [19]. αTAT1 acetylated α-tubulin and is required for ciliogenesis [12], whereas HDAC6 catalyzes the deacetylation of α-tubulins [20]. Therefore, we investigated αTAT1 and HDAC6 expressions and HDAC6 activity in the kidneys. The expression of αTAT1 in the kidneys was significantly increased after HWI compared with NWI (Fig. 2A, B), whereas the expression and activity of HDAC6 were not changed by HWI (Fig. 2A, C, D). Acetylated α-tubulin, but not total α-tubulin, expression in the kidney was greater in HWI than in NWI (Fig. 2E, F). Furthermore, HWI elevated p-ERK1/2 and Exoc5 expressions in the kidneys compared with NWI (Fig. 2G–J). These results indicate that HWI-induced primary cilium elongation is associated with increased αTAT1, p-ERK1/2, and Exoc5 expressions, thereby increasing α-tubulin acetylation and assembly of the microtubule.
U0126 blocks the high water intake-induced primary cilium elongation
Then, we investigated whether U0126, an inhibitor of MEK kinase, blocked HWI-induced primary cilium elongation. U0126 nearly completely blocked HWI-induced increases in p-ERK, αTAT1, and Exoc5 expression (Fig. 3A–E). However, U0126 did not affect the expression and activity of HDAC6 in both NWI and HWI conditions (Fig. 3C, F, G). U0126 blocked HWI-induced increases in α-tubulin acetylation, without a significant change in the total α-tubulin amount (Fig. 3H, I). Furthermore, U0126 blocked the HWI-induced primary cilium elongation without significant cell feature changes (Fig. 4). However, there were no significant morphological changes such as tubule cell expansion and damage (data not shown).
To define the role of ERK on the elongation, we determined that U0126 prevents primary cilia elongation of MDCK cells. U0126 treatment to confluent-grown MDCK cells for 2 days inhibited the elongation of primary cilia (Fig. 5A, B). However, U0126 treatment did not affect Exoc5 expression (Fig. 5C, D). As expected, U0126 inhibited ERK phosphorylation (Fig. 5E, F). These results indicate that the HWI-induced primary cilium elongation is associated with ERK1/2 activation.
High water intake-induced diluted urine production is impeded by U0126
Finally, we assessed the effect of HWI-induced ERK activation on AQP-2 expression and diluted urine production. First, we determined the AQP-2 expression after HWI. Compared with NWI, HWI decreased AQP-2 expression in the kidneys (Fig. 6A, B). In addition, HWI decreased AQP-2 expression in the membrane fraction of the kidneys, leading to an increase in AQP-2 expression in the cytosolic fraction of the kidneys (Fig. 6C–F). U0126 treatment inhibited the HWI-induced decreases in AQP-2 expression in both whole-kidney lysates and membrane fraction of the kidneys (Fig. 6A–D). Furthermore, U0126 prevented the HWI-induced increase in AQP-2 expression in the cytosolic fraction of the kidneys (Fig. 6E, F). In NWI mice, U0126 did not affect the AQP-2 expression in the whole-kidney lysates and fractioned kidney samples compared with vehicles (Fig. 6A–6F). Consistent with the reduction in the membrane fraction, HWI led to decreased AQP-2 expression at the apical plasma membrane with increased cytosolic expression in principal cells (Fig. 6G). U0126 blocked this HWI-induced membrane localization of AQP-2 (Fig. 6G).
Next, we determined the effect of U0126 on HWI-induced urine production. U0126 administration significantly inhibited HWI-induced increases in urine volume, urine Na+ concentration, and glomerular filtration rate (GFR), and decreases in urine osmolality when compared to those in vehicle administration (Table 1). However, U0126 administration in NWI did not induce significant changes in urine volume, urine Na+ concentration, urine osmolality, and GFR when compared with vehicle administration (Table 1). There were no significant changes in water intake amount, food intake amount, and BW in both NWI and HWI mice after U0126 administration. These results indicate that U0126 disturbs the HWI-induced ability of the kidneys to dilute urine, suggesting that ERK activation is a critical process for diluted urine production of the kidneys under HWI conditions.
Discussion
In this study, we report for the first time that HWI induces primary cilium elongation in renal tubular cells through ERK activation and increases αTAT1 and Exoc5 expressions and that ERK inhibition blocks these HWI-induced changes, thereby impeding the kidney’s ability to diluted urine production in response to water intake increase. These data indicate that the alteration in primary cilium length in tubular epithelial cells is an essential process for urine production and provides new insights into how cilia length alteration in renal tubular cells is associated with urine concentration.
The lengths of the primary cilia in renal tubular cells are dynamically altered under diverse pathological and physiological conditions [21–23]. Studies have reported that fluid flow stimulates the change in primary cilium length and that primary cilium length alteration is also required for this loading-induced cellular response to fluid flow changes [24,25]. Espinha et al. [24] reported that fluid flow stimulates the microtubule attachments around the primary cilia anchoring area, which can result in extension of primary cilia. In a recent study, we found that total water intake restriction for 48 hours shortens the primary cilia in the renal tubular cells of mice, producing concentrated urine [8]. By contrast, an increase in urine flow by unilateral nephrectomy elongates the primary cilia in the tubular cells of the remaining kidney [6]. Ichii et al. [26] reported that primary cilium elongation increases the sensitivity of cells to urine flow. In this study, we found that HWI, which increases kidney fluid flow and GFR and produces diluted urine [27], elongates the primary cilia in renal tubular epithelial cells. In the present study, 3% sucrose water intake for 2 days did not induce any significant changes in plasma glucose concentration compared to NWI, suggesting that the cilia elongation in HWI mice may not, or minimally, be associated with blood glucose. Based on previous and present studies, we speculate that increased fluid flow in the kidneys due to HWI stimulates primary cilium elongation and that primary cilium elongation is associated with diluted urine production.
Evidence showed that ciliogenesis is involved in the regulation of microtubule organization in various cells [28]. The assembly and disassembly of microtubules are major processes in the elongation and shortening of the primary cilia, respectively [6,25]. The assembly of microtubules is stimulated by the acetylation of α-tubulins, whereas the disassembly is stimulated by the deacetylation of α-tubulins [5,9,10]. Recent studies have shown that αTAT1, a regulator of α-tubulin acetylation, elongates the primary cilia by increased α-tubulin assembly in microtubules [6,29], whereas the activation and overexpression of HDAC6, an inducer of α-tubulin deacetylation, shorten the primary cilia [30,31]. We previously reported that HDAC6 inhibition prevents the shortening of the primary cilia due to water intake restriction [8]. In this study, we found that HWI increases αTAT1 expression and that HWI increased the acetylated form of α-tubulins. Interestingly, unlike our expectation based on our previous study showing that cilia shortening is associated with lowered HDAC6 activity [8], HWI did not induce significant changes of HDAC6 expression and activity. This phenomenon may be explained by the deferent responses of kidney tubule cells against water restriction and water intake increase and the involvement of variety of factors such as αTAT1. Further studies are required for the clear explanation of this phenomenon. However, our data suggest that increased αTAT1 expression is associated with HWI-induced primary cilium elongation, by increased α-tubulin acetylation, thereby increasing the assembly of microtubules.
Recent studies have demonstrated that the ERK pathway is associated with ciliogenesis [5,28,32]. In the present study, HWI activated ERK, and an ERK inhibitor prevented HWI-induced primary cilium elongation, along with increased α-tubulin deacetylation, and the ERK inhibitor blocked HWI-induced αTAT1 increase, but not HDAC6 inactivation. However, Dougherty et al. [28] reported that ERK inhibition blocked cilia growth by the defect of cilia assembly in hTERT RPE-1 cells and hTERT-immortalized retinal pigment epithelial cells. In the present and previous studies, we found that ERK inhibition by U0126 inhibited primary cilia elongation in the MDCK tubular epithelial cells [5]. In contrast, Wang et al. [32] reported that ERK suppressed ciliogenesis in renal tubular epithelial cells after cisplatin-induced acute kidney injury. This discrepancy (in Wang et al.’s report, ERK suppresses ciliogenesis, whereas in our study, ERK activates ciliogenesis) may be due to experiments. Wang et al. [32] investigated cilia length in renal tubular epithelial cells recovered from severe cisplatin injury, whereas we determined cilia length under hydration conditions that do not induce renal tubule cell damage. ERK is involved in cell differentiation, which affects cilium length [16,33]. Moreover, cilium length is closely related to cell differentiation [25,34]. In the present study, we did not find significant changes in PCNA expression and cell proliferation changes under hydration conditions (data not shown).
We recently found that the overexpression of Exoc5, a critical protein for ciliogenesis, activates ERK in MDCK cells [35], whereas Exoc5 gene deletion in mice and the mutation of the Exoc5 ciliary targeting sequence in MDCK cells shorten the primary cilia in renal tubular epithelial cells [36]. In the present study, we found that U0126 prevented HWI-induced Exoc5 increase. However, U0126 did not affect Exoc5 expression in MDCK cells. These data indicate that ERK activation stimulates HWI-induced primary cilium elongation. However, it is not clear that this ERK-associated cilia elongation is associated with Exoc5 expression. Therefore, to define the precise mechanism of ERK pathways on ciliogenesis, further work is needed to fully control ERK activation and Exoc5 expression.
Recent studies have demonstrated that the ERK pathway is associated with urine concentration in the kidneys by the regulation of AQP-2 expression [37,38]. HWI stimulates diluted urine production by the kidneys by regulating water channel movement from the apical plasma membrane to the cytosol and decrease in tubular cells. AQP-2 expression and apical localization in the kidneys decrease during overhydration and increase after dehydration [39,40]. Consistent with these, in the present study, HWI decreased AQP-2 expression and apical localization in the kidneys, producing diluted urine, and these HWI-induced effects were blocked by pretreatment with an ERK inhibitor. In present study, ERK inhibition prevented HWI-induced increases in urine volume, urine Na+ concentration, and GFR and decreases in urine osmolality. However, HWI and U0126 administration into NWI and HWI did not induce significant changes in plasma osmolality compared to NWI and vehicle-administrated NWI or HWI, respectively. Therefore, we speculate that either those amount of changes of urine volume and urine osmolality induced by HWI and U0126 may not influence the plasma osmolality, or that this 2 days of HWI and U0126 administration may not exceed body fluid maintaining range. These data indicates that ERK activation is linked to diluted urine production, suggesting that ERK inhibition disturbs kidney compensatory response to HWI conditions.
Taken together, although our studies have not provided a precise mechanism for how primary cilium elongation regulates urine concentration, our data clearly show that changes in the primary cilium length are a required response to produce diluted urine under HWI conditions, suggesting that control of primary cilia length could be a tool for the various diseases which are related with abnormal body water and electrolyte balances.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant (NRF-2020R1A2C2006903 to KMP), funded by the Korean government.
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization, Methodology: MJK, KHH, JHL, KMP
Data curation, Formal analysis, Investigation: MJK, SJH, SYS
Funding acquisition: KMP
Writing–original draft: MJK, KHH, JHL, KMP
Writing–review & editing: All authors
All authors read and approved the final manuscript.

