HELLP syndrome in a pregnant patient with Gitelman syndrome
Article information
Abstract
Gitelman syndrome is characterized by hypokalemia, metabolic alkalosis, hypocalciuria, and hypomagnesemia. The clinical course of Gitelman syndrome in pregnant women remains unclear, but it is thought to be benign. We report here the first Korean case of atypical eclampsia in a 31-year-old who was diagnosed with Gitelman syndrome incidentally during an antenatal screening test. The patient did well during pregnancy despite significant hypokalemia. At 33 weeks’ gestation, the patient exhibited eclampsia, hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, and renal insufficiency without significant hypertension or proteinuria. We explain this unusual clinical course through a review of the relevant literature.
Introduction
Gitelman syndrome is an autosomal recessive renal tubular disorder that results in hypokalemia, metabolic alkalosis, hypomagnesemia, hypocalciuria and increased renin and aldosterone levels [1,2]. Clinically, salt craving, nocturia, tetanic episodes, paresthesias, and muscle weakness or paralysis are the most frequent symptoms of Gitelman syndrome. At the molecular level, Gitelman syndrome is caused by an inactivating mutation in the SLC12A3 gene on chromosome 16q13 and results in an abnormality of the thiazide-sensitive sodium chloride co-transporters in distal convoluted tubules [2]. While the effects of Gitelman syndrome on pregnancy remain unclear, most patients have good obstetric and neonatal outcomes despite ongoing hypokalemia and hypomagnesaemia. We describe atypical eclampsia in a pregnant woman with Gitelman syndrome diagnosed incidentally through a screening test during pregnancy.
Case report
A 31-year-old pregnant woman, gravida 2, para 1, in the 11th week of gestation was referred to our nephrology clinic for evaluation of hypokalemia detected incidentally during a routine health exam. She complained of chronic fatigue and recurrent numbness of both her hands. She had no relevant medical history, and denied laxative or diuretic abuse. There was no history of renal disease in her family.
On examination, she was thin (height 160 cm, weight 40 kg) with blood pressure of 90/60 mmHg without significant postural drop and no evidence of dehydration or fluid overload.
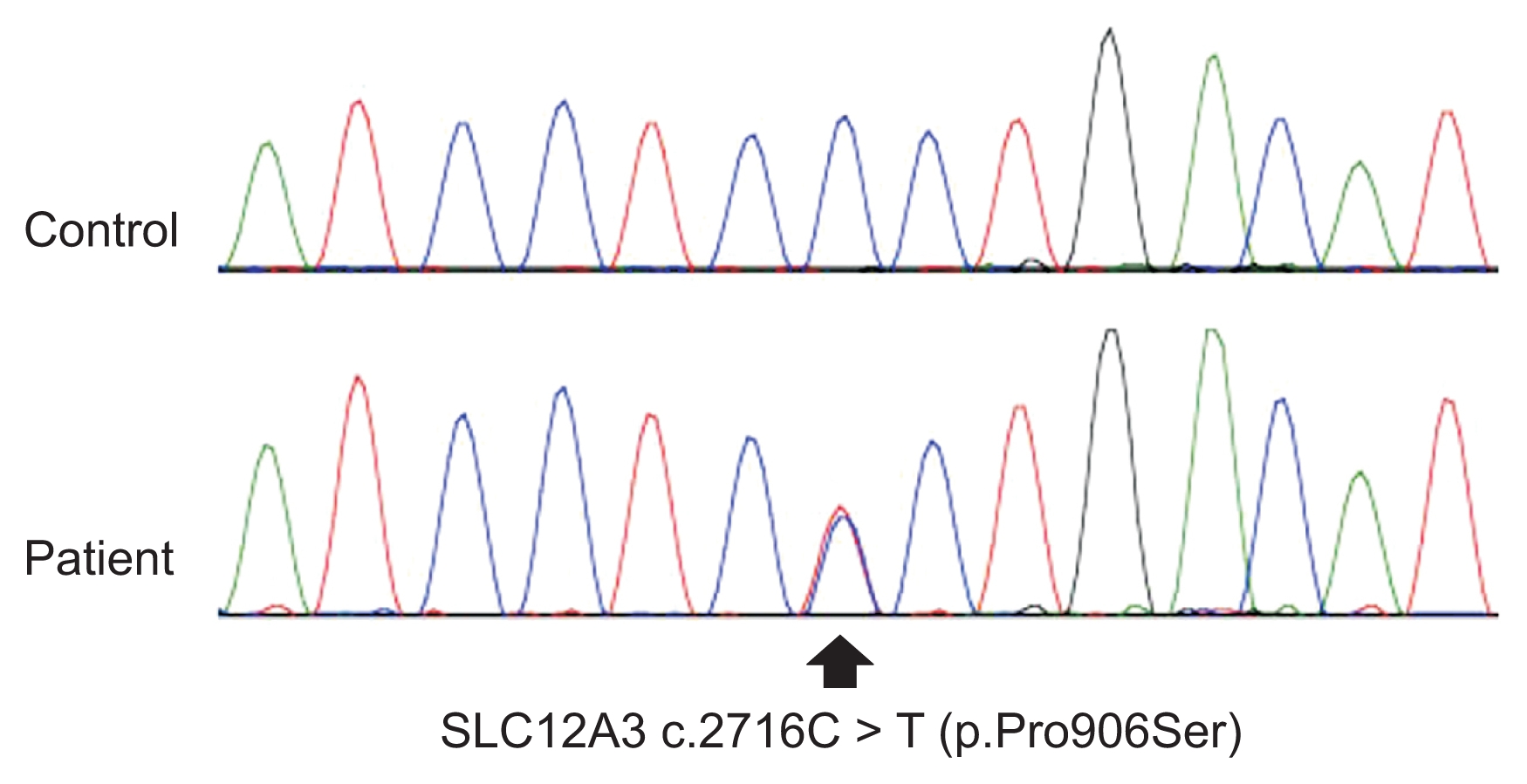
Biochemical investigation revealed hypokalemia with metabolic alkalosis, hypocalciuria, increased plasma re-nin activity and aldosterone (serum K, 2.57 mmol/L; total CO2, 32.3 mmol/L; random urine calcium/creatinine (Cr), 0.00016 mg/mg Cr; plasma renin activity, 10.83 ng/mL/hr; aldosterone, 24.9 ng/dL; respectively). The patient’s transtubular potassium gradient (TTKG) was elevated despite hypokalemia (TTKG, 8.25). Serum magnesium level was within normal range (2.11 mg/dL) (Table 1). Neither proteinuria (33 mg/gCr) nor hematuria was detected. These findings suggested Gitelman syndrome, with the exception of the normal magnesium level. The diagnosis was confirmed when a mutation c.2716C>T (p.Pro906Ser) (heterozygote) in the SLC12A3 gene was found on genetic analysis (Fig. 1). Oral potassium supplementation was started, and the patient underwent routine examination during her pregnancy (Fig. 2). Spironolactone was not given because of the possible antiandrogenic effects on the male fetus.
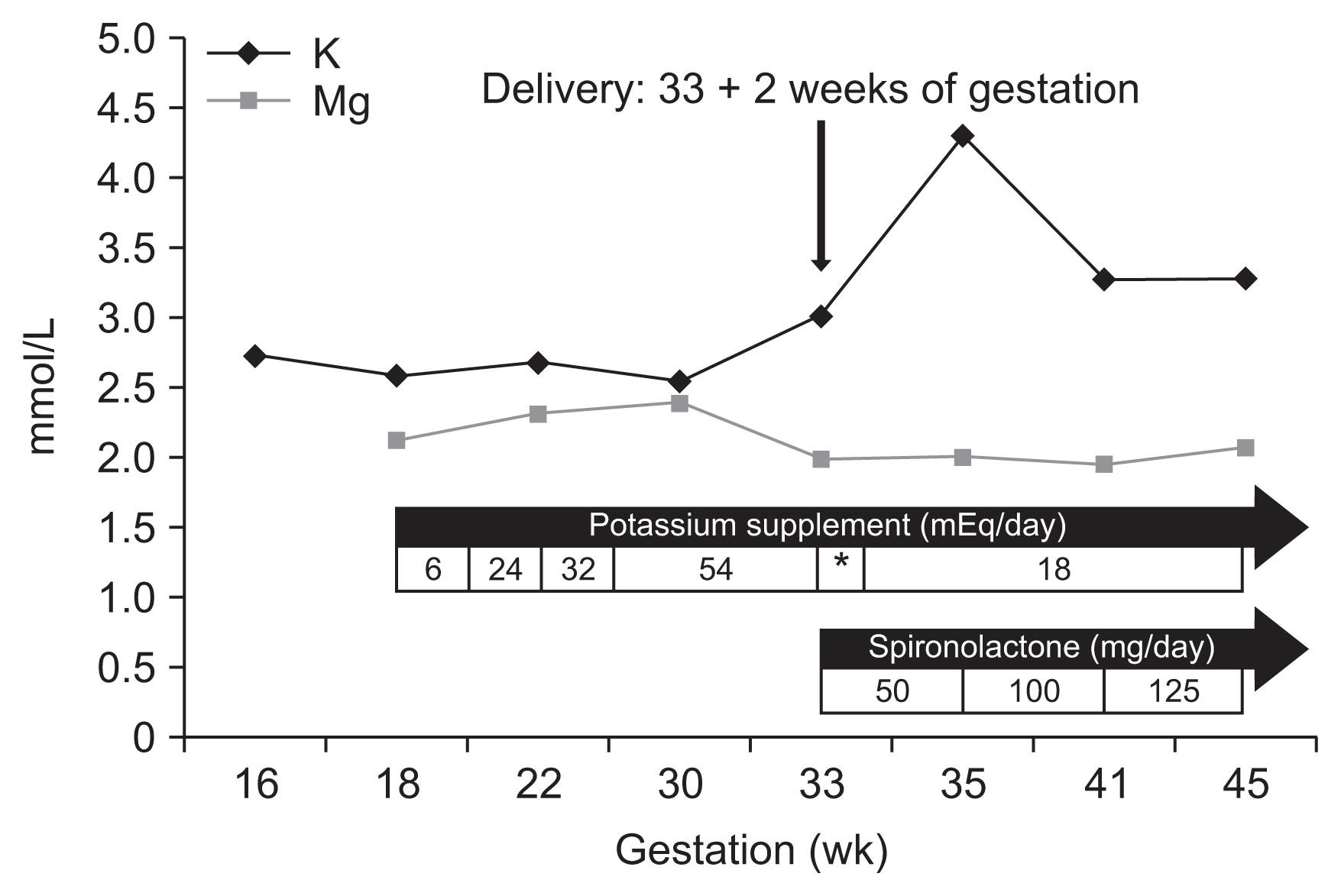
Evolution of serum potassium during pregnancy and after delivery in our patient according to supplements and medication administered.
*On the day of delivery and the next day, intravenous potassium and intravenous magnesium were supplied.
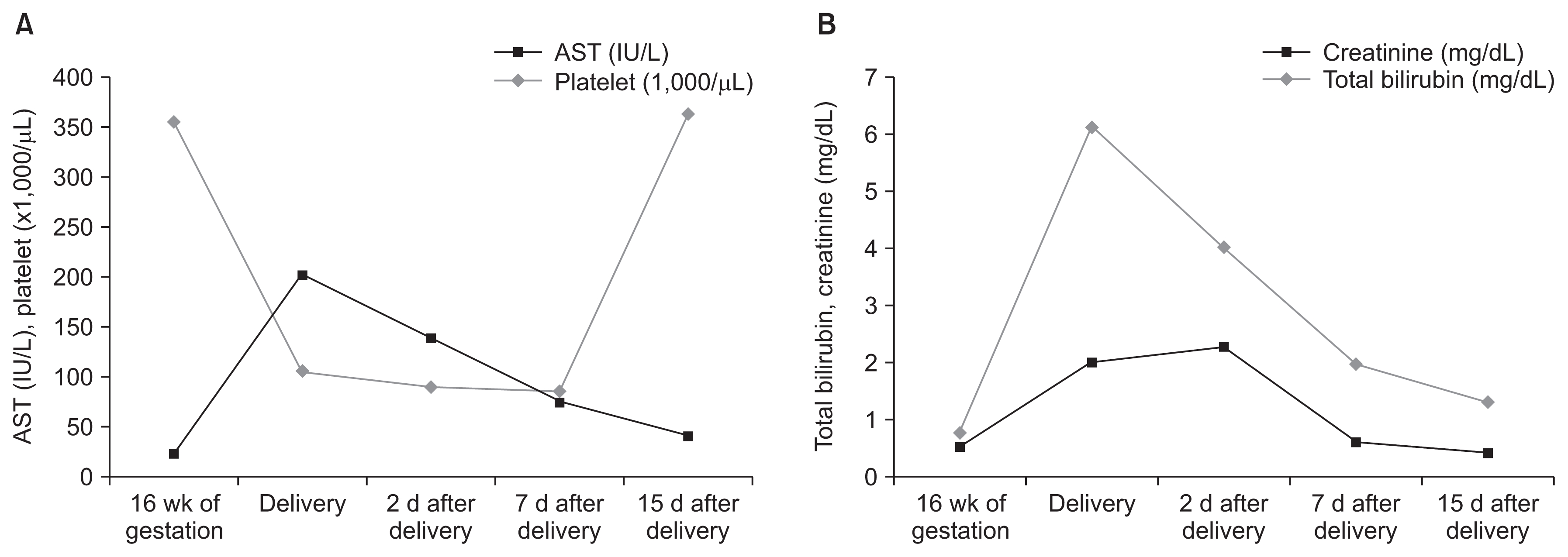
When the patient was at 33 weeks’ gestation, she visited the emergency department with a tingling sensation and tetany in both the upper and lower extremities. Her blood pressure was 120/80 mmHg, within normal range, but higher than her usual blood pressure (90/60 mmHg). Her Cr level was elevated (2.03 mg/dL) and renal protein excretion was slightly increased (0.2 g/gCr). Additional laboratory results were compatible with HELLP (hemolysis, elevated liver enzyme, low platelet) syndrome, including decreased haptoglobin (8 mg/dL) and increased plasma hemoglobin (12.5 g/dL) levels, increased lactic acid dehydrogenase (969 IU/L) levels, schistocytes on peripheral blood smears, elevated liver enzymes (aspartate aminotransferase, 210 IU/L; alanine transaminase, 108 IU/L), and decreased platelet counts (101,000/mm3). Fractional excretion of sodium was 0.72% and serum uric acid was 23.86 mg/dL.
The patient had abnormal uterine contractions resulting in spontaneous vaginal delivery of a male infant weighing 1,630 g (50% for his gestational age). His Apgar score was 4 in the first minute and 5 after five minutes, and he was admitted to the neonatal intensive care unit. Hyperbilirubinemia (peak total bilirubin 9.87 mg/dL, indirect bilirubin 1.03 mg/dL) was detected, but his electrolytes were normal other than mild hyponatremia (sodium, 126.7 mmol/L; potassium, 3.01 mmol/L; chloride, 88.8 mmol/L). Postpartum, the patient suffered from disseminated intravascular coagulation (platelets, 89,000/μL; prothrombin time, 9.9 sec; activated partial thromboplastin time, 32.4 sec; D-dimer, 2.60 μg/mL; fibrin degradation products 8.1 μg/mL; and antithrombin III, 22%). The patient and neonate recovered slowly during the following weeks (Fig. 3). The patient was discharged on daily oral potassium and spironolactone (Fig. 2). Outpatient follow-up after five months revealed patient blood pressure of 90/60 mmHg and serum potassium and magnesium levels of 3.03 mmol/L and 2.17 mg/dL, respectively.
Discussion
The clinical course of Gitelman syndrome in pregnant women remains unclear. A thorough review of the literature demonstrated a few reports on the impact of Gitelman syndrome on pregnancy [3–8], but most did not describe mortalities or morbidities in the course of Gitelman syndrome, and no adverse fetal outcomes directly related to Gitelman syndrome were reported. Oligohydramnios, intrauterine growth retardation, severe cramping due to hypokalemia and hypomagnesemia, Sheehan’s syndrome, gestational diabetes, miscarriages in the first trimester, premature delivery, polyhydramnios, preeclampsia and placental abruption have been reported in patients with Gitelman syndrome [9]. While there was a fluid deficit and electrolyte imbalance in the abovementioned case, it remains unclear whether any of these complications were directly related to Gitelman syndrome [9].
In our case, the patient was diagnosed with Gitelman syndrome based on a routine check-up. As demonstrated, Gitelman syndrome can appear with a normal serum magnesium level [10]. The patient in the present case experienced a normal pregnancy until 33 weeks’ gestation, despite significant hypokalemia. At 33 weeks’ gestation, she experienced sudden tetany across her whole body. We initially presumed it was caused by hypokalemia aggravation. However, her potassium level was higher (3.5 mmol/L) than usual, and HELLP syndrome, renal insufficiency, generalized edema and hypoalbuminemia appeared. These clinical features were compatible with a diagnosis of eclampsia with HELLP syndrome and renal insufficiency [11]. The blood pressure of the patient did not satisfy the diagnostic criteria for preeclampsia (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg), even though it was significantly higher (120/80 mmHg) than previous measurements in this patient (90/60 mmHg). A mild elevation in protein urine excretion also appeared (0.2 g/gCr).
Blood pressure in Gitelman syndrome is usually low because of salt loss associated with mutations in the SLC12A3 gene (a Na-Cl cotransporter) [12]. Eclampsia is defined as the occurrence of unexplained seizure during pregnancy in women with preeclampsia [11]; however, in some studies, up to 40% of eclampsia patients experienced seizure at a normal blood pressure without proteinuria [13]. Close monitoring is also recommended if the blood pressure of a pregnant woman increases more than 30 mmHg in systolic pressure or 15 mmHg in diastolic pressure, even though this is insufficient evidence for a diagnosis of preeclampsia (criteria: higher than 140 mmHg systolic pressure and 90 mmHg diastolic pressure) [14]. It is unclear whether eclampsia, HELLP syndrome, and renal insufficiency in this patient were caused by Gitelman syndrome. However, the tendency to lose salt and water in Gitelman syndrome might have inhibited the patient’s blood pressure from rising to meet the diagnostic criteria. Risk factors of eclampsia including primiparity, previous preeclamptic pregnancy, chronic hypertension, chronic renal disease, a history of thrombophilia, multifetal pregnancy, in vitro fertilization, a family history of preeclampsia, diabetes mellitus, obesity, systemic lupus erythematous and advanced maternal age [11] were not observed in this patient.
We comprehensively reviewed the English literature for cases of Gitelman syndrome in pregnant women. The characteristics of 15 patients, including this case, are summarized in Table 2. Oligohydramnios was the most common complication. Chronic urinary water and electrolyte loss might lead to oligohydramnios. The prognosis of infants in these cases was good. In the present case the neonate had neonatal jaundice, but this improved within two weeks.
Normalization of serum potassium and magnesium levels is difficult to achieve in pregnant patients with Gitelman syndrome. In normal pregnant women, despite electrolyte abnormalities caused by the fetal need for electrolytes and elevated levels of aldosterone and other mineralocorticoids, decreases in potassium level are rare because of the protective effect of high progesterone [15]. In Gitelman syndrome, hypokalemia can be aggravated in pregnancy because the antikalliuretic effect of progesterone does not allow for sufficient compensation. Additionally, in active labor, increased beta adrenergic tone may cause a shift in extracellular potassium in the intracellular space and can aggravate hypokalemia and clinical symptoms of Gitelman syndrome [4]. In spite of this, the majority of cases report uncomplicated maternal and fetal outcomes. The patient in this case was asymptomatic even though her potassium level was less than 3 mmol/L.
The management of patients with Gitelman syndrome has included the use of potassium-sparing diuretics and potassium/magnesium supplements. Because spironolactone is classified as a category C material by the US Food and Drug Administration, it is not routinely used in pregnant women with Gitelman syndrome, although no feminization has been reported in male newborns in such cases [6]. The patient in this case refused spironolactone because of the potential adverse fetal effects. Amiloride and eplerenone are both class B drugs and can be used in Gitelman syndrome during pregnancy [4]. Angiotensin-converting enzyme inhibitors are contraindicated in pregnancy due to teratogenic effects and impaired fetal growth.
In summary, Gitelman syndrome appears to have benign effects on pregnancy in most cases, but the present patient experienced eclampsia, HELLP syndrome, and renal insufficiency despite the absence of significant hypertension or proteinuria. Awareness that an unusual clinical presentation of pregnancy complication can be associated with abnormal.
Acknowledgments
The authors would like to thank Chang-Seok Ki and Ju Sun Song (Samsung Medical Center) for genetic analysis and chromatography.
This study was supported by the National Research Fo-oundation of Korea (NRF) funded by the Ministry of Education (No. 2016914796).
Notes
Conflicts of interest
The author has no conflicts of interest to declare.