Evaluating the association of interleukin-10 gene promoter -592 A/C polymorphism with lupus nephritis susceptibility
Article information
Abstract
Background
Interleukin-10 (IL-10) is an important immunoregulatory cytokine. There are few studies evaluating the association between IL-10 and lupus nephritis (LN). The aim of this study was to evaluate the association of IL-10 gene promoter -592 A/C with LN susceptibility.
Methods
The study was conducted on 84 patients with systemic lupus erythematosus (SLE). Patients were divided into LN group (Group I, 48 patients) and non-LN group (Group II, 36 patients). The -592 A/C polymorphisms in IL-10 promoter gene were determined by polymerase chain reaction and restriction fragment length polymorphism in both groups. IL-10 was determined by enzyme-linked immunosorbent assay. Frequencies of the genotypes were compared between LN and non-LN patients and among LN patients with different pathologic classes.
Results
There was a significant increase in serum level of IL-10 (P = 0.001) in Group I compared with Group II and significant positive correlation between serum IL-10 and SLE disease activity index (r = 0.466, P = 0.001) in Group I. There were no significant differences in the distribution of the IL-10 gene promoter -592 A/C genotypes or the allele frequencies between Groups I and II. There was no significant difference between AC/CC and AA genotypes with SLE disease activity index, proteinuria, hematuria, anti-double-stranded DNA, and IL-10 in Group I. There was no significant difference in the distribution of AC and CC genotypes among different pathologic LN classes.
Conclusion
IL-10 suggested to play a role in pathogenesis and development of LN. However, the promoter -592 A/C of IL-10 gene suggested to be not associated with serum IL-10 levels or LN susceptibility. In addition, it appears that promoter -592 A/C of IL-10 gene not associated with LN activity or the pathologic classes of LN.
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease. Many factors are associated with the development of the SLE, including genetic, ethnic, immunoregulatory, hormonal, and environmental factors [1], [2], [3], [4].
The role of genetics in the development of SLE is supported by that SLE is more common in first-degree relatives of patients with SLE (familial prevalence, 10–12%). Prevalence rates are higher in monozygotic twins (24–58%) than in dizygotic twins (2–5%) [1], [2], [3], [4].
The major and serious manifestation of SLE is lupus nephritis (LN). In most patients with SLE, LN is histologically evident, and kidney biopsy should be considered in any patient with SLE who has clinical or laboratory evidence of active nephritis, especially on the first episode of nephritis [5].
Many studies suggest that genetic predisposition plays an important role in the development of both SLE and LN. Multiple genes, many of which are not yet identified, mediate this genetic predisposition [1], [2], [3], [4], [6], [7], [8], [9] .
Interleukin (IL)-10 is a cytokine with pleiotropic effects in immunoregulation and inflammation and produced mainly by monocytes and B lymphocytes [10], [11]. IL-10 promotes B-cell-mediated functions, enhancing survival, proliferation, differentiation, and antibody production [12], [13]. It also inhibits T cell function by suppressing the expression of proinflammatory cytokines such as tumor necrosis factor-α, IL-1, IL-6, IL-8, and IL-12 [14]. It also inhibits antigen presenting cells by downregulating major histocompatibility complex Class II and B7 expression [14], [15], [16], [17], which in SLE may contribute to impaired cell-mediated immunity.
In humans, the IL-10 gene is located on chromosome-1 and its receptor is located on chromosome 11 [18]. The IL-10 gene encodes for 5 exons. The IL-10 promoter is highly polymorphic, and in this region, 2 CA-repeat microsatellites (IL-10.G and IL-10.R) and 3 single nucleotide polymorphisms (SNPs), at positions –1082, –819, and –592 from the transcription start site, have been identified to correlate with IL-10 production [10]. Haplotypes comprising 3 SNPs at positions –1082, –819, and –592 have also been found to correlate with IL-10 serum level [10].
There are several studies suggesting that the IL-10 gene is associated with SLE susceptibility [19]. Studies in lupus animal models and humans have shown that anti-IL-10 treatment can decrease disease activity in terms of clinical features and biologic markers [20], [21], [22]. Interestingly, Llorente et al [23] demonstrated that IL-10 production by monocytes and B cells in healthy members of families with SLE was significantly higher than that of healthy unrelated controls, but was similar to that of SLE patients, thus suggesting that a genetically controlled high innate IL-10 production may predispose to SLE development [23]. Although there are several studies evaluating the association between IL-10 and SLE, the studies evaluating the association between IL-10 and LN are few.
Aim of the study
The aim of this study was to determine the distribution of the promoter -592 A/C of IL-10 gene in Egyptian patients with SLE and LN and evaluate the role of the promoter -592 A/C of IL-10 gene in the pathogenesis and clinical and histopathologic classes of LN.
Methods
This study was conducted on 84 patients with SLE who have the criteria of Systemic Lupus International Collaborating Clinics group [24]. Patients were divided into LN group (Group I, 48 patients with mean age 29.63 ± 8.91 years) and non-LN group (Group II, 36 patients with mean age 31.81 ± 0.20 years). The patients of both groups were matched for age, gender, and ethnic origin. LN was diagnosed clinically by the presence of persistent proteinuria or hematuria and confirmed by kidney biopsy. Non-LN patients were diagnosed according to the criteria of Systemic Lupus International Collaborating Clinics group [24], including arthritis, skin rash, positive antinuclear antibodies (ANAs), and positive anti-double-stranded DNA (dsDNA), but without renal involvement in the form of proteinuria, hematuria, or abnormal renal functions. SLE patients with proteinuria other than LN as pregnancy and fever or patients with impaired renal function due to any other cause than LN as diabetic nephropathy and patients with history of renal trasnsplantation or hepatitis C virus and hepatitis B virus and other connective tissue diseases other than SLE were excluded from the study. All these patients were selected from the nephrology outpatient clinics in nephrology departement, Theodor Bilharz Research Institute, Cairo, Egypt. The study was approved by the appropriate ethics committee and has therefore been performed in accordance with Declaration of Helsinki, and written informed consent was obtained from each patient participated in the study.
Each patient underwent thorough history taking and complete clinical examination.
Peripheral venous blood samples were collected from patients after proper disinfection.
(1) Two milliliters on EDTA for complete blood count.
(2) Five milliliters of blood in a plain glass tube left to clot at room temperature for 30 minutes then centrifuged for 10 minutes to obtain serum for chemical and immunological tests.
Routine examinations included urine analysis, renal function tests (serum creatinine, urea, sodium, potassium, and uric acid), complete blood count, erythrocyte sedimentation rate, and C-reactive protein. Serum C3 and C4, ANA, and anti-dsDNA were also conducted.
ANA was measured using indirect immunoflourescence. Anti-dsDNA were measured using solid-phase enzyme immunoassays kits. C3 and C4 were measured using Nephelometer (BN ProSpec, Dade Behring, Marburg, Germany).
Determination of -592 A/C polymorphisms in the IL-10 gene promoter
Genomic DNA was extracted from EDTA–whole blood using a phenol chloroform extraction method. The -592 A/C polymorphism in the IL-10 gene promotor was determined by polymerase chain reaction (PCR) and restriction fragment length polymorphism protocol using the following designed primer sequences:
5′ TCC AGC CAC AGA AGC TTA CAA C 3′ (forward);
5′ AGG TCT CTG GGC CTT AGT TTC C 3′ (reversed).
PCR was performed on a Gene-Amp PCR System 2700 (Applied Biosystems, Foster City, CA, USA) in the following conditions: 94°C for 5 minutes; 35 cycles of 94°C for 30 seconds, 64°C for 45 seconds, and 72°C for 60 seconds; followed by a final extending step at 72°C for 10 minutes. The PCR product was digested for 4 hours at 37°C with the restriction enzyme Rsa I (New England Biolabs, Ipswich, MA, USA). The genotypes of IL-10 -592 A/C were distinguished by electrophoresis separation of the fragments on a 3% agarose gel with 0.1% ethidium bromide to visualize under ultraviolet light.
IL-10 was determined by enzyme-linked immunosorbent assay (ELISA) using an ELISA kit (AviBion human IL-10 ELISA kit; Orgenium Laboratories, Finland) [25]. The mean value of serum IL-10 levels was compared statistically between LN and non-LN groups and between LN patients with normal serum creatinine (20 patients) and LN patients with high serum creatinine (28 patients).
Kidney biopsy was performed for Group I patients and classified according to International Society of Nephrology/Renal Pathology Society [26]. Disease activity was assessed by SLE disease activity index (SLEDAI) [27], and their correlations with serum IL-10 and promoter -592 A/C of IL-10 gene were analyzed.
Statistical analysis
Results were analyzed as means ± SD or number (%). Comparison between different parameters in the 2 studied groups was performed using unpaired 2-tailed Student’s t tests (GraphPad QuickCalcs). Comparison between categorical data was performed using Chi-square test. Correlation between different parameters in the cases group was performed using Pearson’s correlation (MedCal Statistical Software). Statistical analysis was performed with the aid of the SPSS computer program (version 12 windows). The data were considered significant if P < 0.05 and highly significant if P < 0.01.
Results
Demographic and clinical characteristics of the studied groups are shown in Table 1, where there was no statistically significant difference between LN group (Group I) and non-LN group (Group II) regarding age, gender, duration of the disease, arthritis, and skin rash, whereas edema of lower limbs and hypertention were statistically significantly high in Group I compared with Group II (P = 0.001).
Laboratory parameters of the studied groups are also shown in Table 1, where there was a statistically significant increase in anti-dsDNA, in Group I (174.11 ± 149.52 IU/mL) compared with Group II (58.21 ± 23.47 IU/mL, P = 0.001). On the other hand, there was a statistically significant decrease in C3 (P = 0.001) and C4 (P = 0.001) in Group I compared with Group II.
There was a statistically significant increase in the mean value of SLEDAI in Group I (14.60 ± 8.63) compared with Group II (10.17 ± 3.11, P = 0.003).
There was a statistically significant increase in the mean value of serum IL-10 in Group I (21.13 ± 14.17 pg/mL) compared with Group II (4.96 ± 3.81 pg/mL, P = 0.001). There was no statistically significant difference in the mean value of serum IL-10 in LN patients with high serum creatinine compared with LN patients with normal serum creatinine (23.11 ± 16.15 vs. 19.15 ± 12.19, P = 0.361).
There was a statistically significant negative correlation between serum IL-10 level and serum C3 (r = –0.512, P = 0.024), and a statistically significant positive correlation between serum IL-10 and SLEDAI (r = 0.466, P = 0.001; Fig. 1) in LN group.
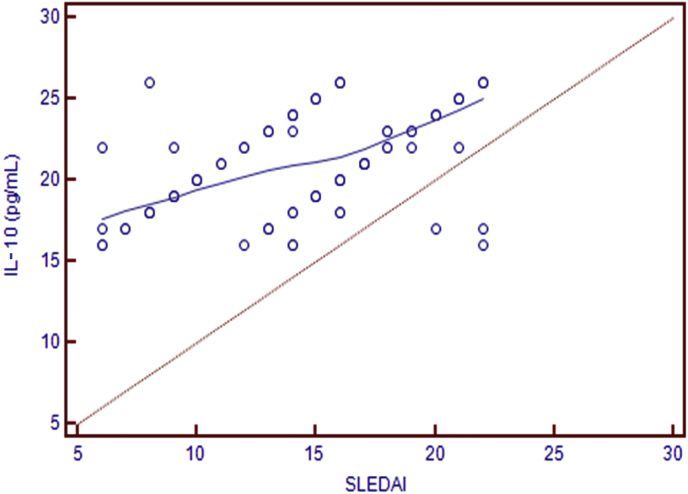
Correlation between IL-10 (pg/mL) and SLEDAI (r = 0.466, P = 0.001).
IL, interleukin; SLEDAI, systemic lupus erythematosus disease activity index.
There were no statistically significant differences in the distribution of the -592 A/C genotypes in IL-10 gene promoter or the allele frequencies between Groups I and II (Table 2).

Distribution of the IL-10 gene promoter -592 A/C polymorphisms and allele frequency in the 2 studied groups
There was no statistically significant difference between AC/CC and AA genotypes with different clinical and laboratory parameters in Group I (Table 3).
Renal pathology data
Fourteen patients (29.16%) were classified as focal proliferative glomerulonephritis (Class III LN), 18 patients (37.5%) were classified as diffuse proliferative glomerulonephritis (Class IV LN), 8 patients (16.7%) were classified as membranous nephropathy (Class V LN), 4 patients (8.3%) were classified as Class IV + V, and 1 patient (2.1%) was classified as sclerosing glomerulonephritis (Class VI LN).
There was no statistically significant difference between AC and CC genotypes with different pathologic classes of LN (AA was not included due to its small number; Table 4).
Discussion
IL-10 gene polymorphisms and increased IL-10 production have been suggested to play a role in susceptibility or exacerbating of SLE [28], [29].
Although LN is one of the major and serious manifestations of SLE, there have been few studies to evaluate the association between IL-10 and LN [16]. In this exploratory study, we evaluated the association of IL-10 and IL-10 gene promoter -592 A/C with LN susceptibility, activity, and histopathologic classes of LN.
The present study showed that serum IL-10 was statistically significantly high in the LN group compared with non-LN group, and there was a positive correlation between IL-10 and SLEDAI and a negative correlation between IL-10 and C3 in LN group. These results are in agreement with the results that are showed by Lit et al [30] and Park et al [31]. The results of Lit et al and Park et al and our results indicate that dysregulation and high levels of IL-10 may play an important role in the pathogenesis and development of LN.
However, the present study found that there was no statistically significant difference in the distribution of the IL-10 gene promoter -592 A/C genotypes between LN patients and non-LN patients suggesting that the -592 A/C polymorphism in the IL-10 gene promoter may not be associated with LN susceptibility and this is similar to the results revealed by Zhu et al [32]. In contrast to our results, in the Hong Kong Chinese population, there was a significant difference in the distribution of the IL-10 gene promoter -592 A/C genotypes between SLE patients with and without renal involvement [33]. These different findings may be explained by the presence of a significant racial variation in the distribution of the -592 A/C polymorphism in the IL-10 gene promoter.
In addition, this study showed that there was no statistically significant difference in the level of IL-10 in AC/CC genotypes compared with AA genotype which is similar to the results showed by Zhu et al [32]. In addition, present study found that there was no statistically significant increase in SLEDAI, anti-dsDNA, proteiuria, hematuria, and urinary casts in AC/CC genotypes compared with AA genotype. In opposition to our results, Zhu et al noted that patients with AC/CC genotypes showed statistically significant increase in SLEDAI, anti-dsDNA, proteiuria, hematuria, and casts compared with AA genotype patients.
As regard the association of the IL-10 gene promoter -592 A/C polymorphism with different pathologic classes of LN, the present study showed that there was no statistically significant difference in the distribution of AC and CC genotypes (AA was not included because of its small number) among different classes of LN (III, IV, and V). However, Zhu et al [32] showed that patients with Class IV LN had a higher frequency of AC/CC genotypes than those with Class V LN suggesting that the IL-10 gene promoter -592 A/C polymorphism may play a role in diverse renal pathologic changes in LN patients and that those carrying a higher frequency of AC/CC genotypes were more likely to have diffuse proliferative lesions in the kidney.
It still remains to be determined how the genetic polymorphism not associated with serum IL-10 level may have an impact on renal lesions of LN. One study showed that the 592 C allele was associated with a higher frequency of positive anti-dsDNA, which have been considered to be responsible for the initiation of LN [32]. This is in opposition to our results as -592 C allele was not associated with a higher frequency of positive anti-dsDNA. IL-10 gene polymorphism might possibly impact on the local IL-10 level in the glomeruli, other than the serum IL-10 level, that could be responsible for the renal lesions in LN and the difference among different pathologic classes [34].
It is also possible that the promoter -592 A/C of IL-10 gene may act indirectly through linkages with some other single nucleotide polymorphisms in IL-10 gene promoter at positions –1082 and –819 that have been identified to correlate with IL-10 production [10]. Haplotypes comprising SNPs at positions –1082 and –819 have also been found to correlate with IL-10 serum level [10]. In addition, SNPs at positions –1082 and –819 have been identified to play a role in the pathologic lesions in the lupus nephritis [32].
This study has some limitations such as small samples of patients, no pathologic data for Group II and there is no follow-up for both groups of patients. So we need larger studies with a follow-up of the patients to detect the treatment response and its effect on the serum levels of IL-10.
In conclusion, IL-10 suggested to play a role in pathogenesis and development of LN. However, the promoter -592 A/C of IL-10 gene suggested to be not associated with serum IL-10 levels or LN susceptibility. In addition, it appears that the promoter -592 A/C of IL-10 gene is not associated with LN activity or the pathologic classes of LN.
Conflicts of interest
All authors have no conflicts of interest to declare.


