| Kidney Res Clin Pract > Epub ahead of print |
Abstract
Background
Methods
Results
Supplementary Materials
Notes
Acknowledgments
Figure 1.
Flow chart of study participant enrollment, randomization, and analysis.
Figure 2.
Changes of muscle strength and physical performance.
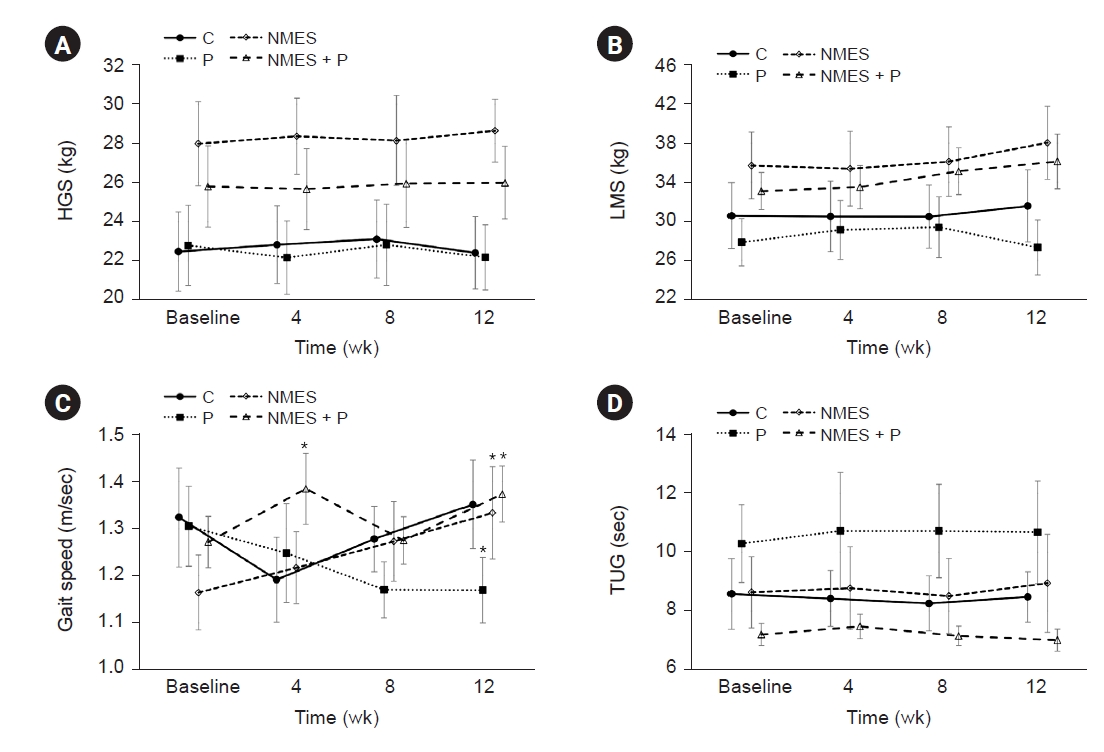
Figure 3.
Comparison of muscle strength and physical performance between the low- and high-NMES intensity groups.
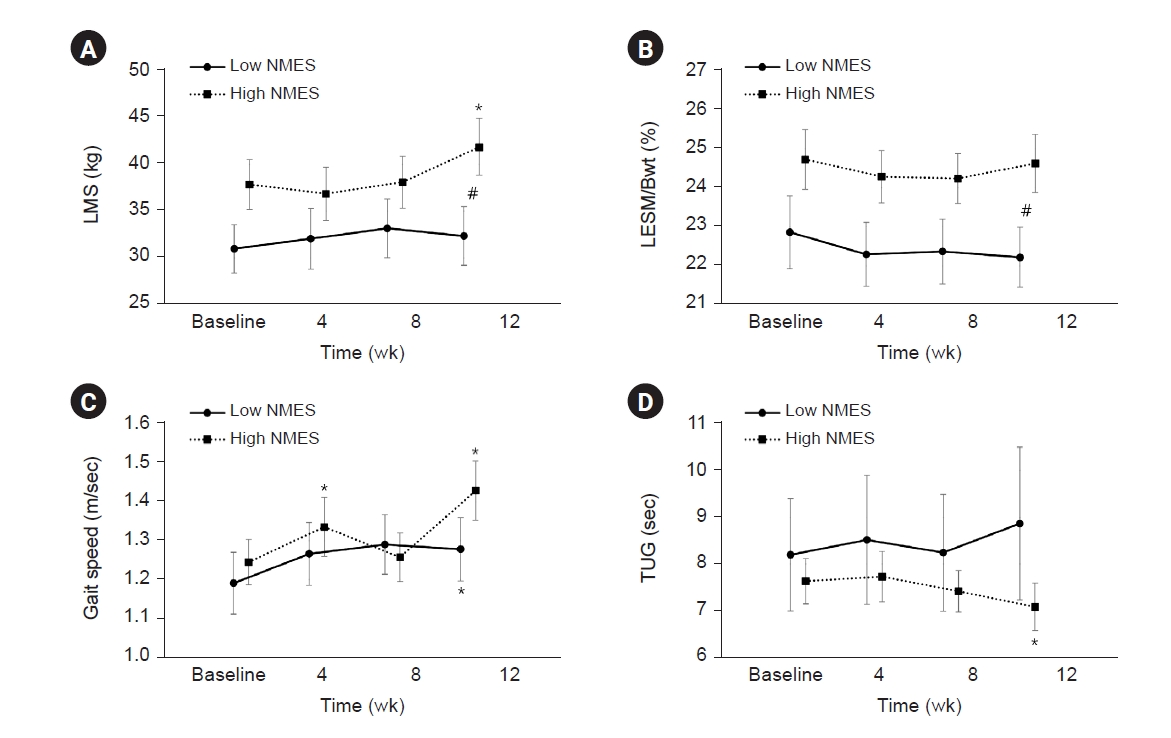
Table 1.
Data are expressed as mean ± standard deviation or number (%). The p-values were tested with analysis of variance for continuous variables or Kruskal-Wallis test, and Pearson chi-square test or Fisher exact test was used to analyze categorical variables.
DM, diabetes mellitus; ESRD, end-stage renal disease; HbA1c, hemoglobin A1c; HD, hemodialysis; hs-CRP, high sensitivity C-reactive protein; iPTH, intact parathyroid hormone; NMES, neuromuscular electrical stimulation; NMES + P, neuromuscular electrical stimulation combined with protein supplementation; nPCR, normalized protein catabolic rate; P, protein supplementation.
Table 2.
| Scale |
Baseline |
12 wk |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | P | NMES | NMES + P | Control | P | NMES | NMES + P | |
| Short form-6 scale | ||||||||
| Physical functioning | 49.0 ± 38.0 | 55.4 ± 38.3 | 61.7 ± 32.6 | 79.0 ± 26.4 | 46.0 ± 31.3ab | 43.9 ± 31.6a | 66.3 ± 33.1a | 78.0 ± 20.3a |
| RP | 63.3 ± 47.1 | 80.4 ± 36.9 | 66.7 ± 45.0 | 51.7 ± 47.7 | 86.7 ± 28.1 | 78.6 ± 39.0 | 70.0 ± 42.5 | 81.7 ± 35.9a |
| Bodily pain | 90.3 ± 17.0 | 94.5 ± 8.27 | 89.0 ± 17.5 | 83.7 ± 21.9 | 95.0 ± 12.4 | 81.8 ± 18.3b | 87.3 ± 16.8 | 83.2 ± 19.7 |
| General health | 49.7 ± 17.2 | 48.6 ± 18.4 | 42.0 ± 22.8 | 32.7 ± 19.4 | 43.7 ± 20.0 | 36.8 ± 16.5 | 44.7 ± 14.1 | 38.3 ± 21.8 |
| Vitality | 50.7 ± 14.1 | 52.5 ± 11.6 | 53.0 ± 20.7 | 49.7 ± 15.9 | 49.7 ± 14.7 | 47.5 ± 7.00 | 58.3 ± 14.6 | 52.3 ± 18.3 |
| Social functioning | 95.8 ± 10.2 | 92.9 ± 11.7 | 93.3 ± 20.0 | 85.8 ± 17.6 | 96.7 ± 8.80 | 80.4 ± 21.2 | 91.7 ± 13.9 | 84.2 ± 22.9 |
| RE | 60.0 ± 47.5 | 78.6 ± 38.4 | 73.3 ± 45.8 | 60.0 ± 47.7 | 88.9 ± 30.0 | 64.3 ± 46.2 | 71.1 ± 41.5 | 86.7 ± 27.6b |
| Mental health | 60.0 ± 15.3 | 63.7 ± 11.4 | 59.5 ± 15.8 | 61.9 ± 14.7 | 59.7 ± 11.8 | 56.3 ± 5.76b | 61.9 ± 9.30 | 61.6 ± 13.4 |
| Overall health rating | 81.1 ± 28.8 | 77.4 ± 28.2 | 90.0 ± 16.4 | 76.7 ± 19.7 | 87.8 ± 19.4 | 78.6 ± 31.6 | 88.9 ± 13.6 | 74.4 ± 28.8 |
| Physical component scale | 42.4 ± 6.95 | 45.3 ± 7.65 | 43.9 ± 8.86 | 42.5 ± 8.24 | 43.6 ± 5.90 | 41.7 ± 4.54 | 44.7 ± 8.78 | 45.4 ± 6.40 |
| Mental component scale | 46.3 ± 8.99 | 47.1 ± 6.87 | 47.3 ± 9.16 | 44.2 ± 8.73 | 47.8 ± 5.30 | 42.8 ± 7.79 | 46.7 ± 7.28 | 46.4 ± 7.80 |
| KD-specific scale | ||||||||
| Symptoms/problems | 93.5 ± 3.76a | 91.2 ± 6.70a | 87.2 ± 9.92a | 84.6 ± 12.4a | 90.0 ± 11.7 | 87.6 ± 5.69 | 89.4 ± 8.39 | 86.8 ± 11.4 |
| KD effects | 72.9 ± 18.5 | 72.5 ± 17.6 | 70.4 ± 19.0 | 67.7 ± 14.2 | 77.3 ± 15.8 | 70.9 ± 19.2 | 76.9 ± 19.2 | 76.5 ± 14.6b |
| KD burden | 46.7 ± 33.1 | 50.0 ± 28.8 | 38.8 ± 25.8 | 35.0 ± 28.0 | 50.4 ± 24.3 | 39.3 ± 26.2 | 50.0 ± 24.3 | 40.4 ± 28.2 |
| Work status | 10.0 ± 20.7 | 25.0 ± 42.7 | 23.3 ± 37.2 | 23.3 ± 32.0 | 20.0 ± 31.6 | 21.4 ± 37.8 | 26.7 ± 37.2 | 20.0 ± 31.6 |
| Cognitive function | 95.1 ± 6.41 | 88.1 ± 12.6 | 93.8 ± 7.75 | 88.4 ± 15.0 | 91.6 ± 12.2 | 83.8 ± 17.5 | 90.2 ± 17.3 | 87.1 ± 15.8 |
| Quality of social interaction | 72.4 ± 12.6 | 68.6 ± 10.6 | 66.7 ± 5.04 | 67.1 ± 17.9 | 72.4 ± 11.8 | 67.6 ± 9.38 | 67.1 ± 9.25 | 69.3 ± 14.0 |
| Sleep | 57.5 ± 20.0 | 61.3 ± 14.9 | 67.8 ± 18.9 | 59.5 ± 16.6 | 56.0 ± 17.2 | 58.0 ± 13.9 | 57.5 ± 19.9 | 63.8 ± 17.9 |
| Social support | 58.9 ± 27.4 | 64.3 ± 24.3 | 58.9 ± 22.6 | 68.9 ± 23.5 | 70.0 ± 9.34 | 72.6 ± 14.0 | 65.6 ± 24.0 | 66.7 ± 21.8 |
| Overall health rating | 62.7 ± 18.7 | 58.6 ± 22.1 | 62.0 ± 19.3 | 48.7 ± 21.7 | 67.3 ± 18.3 | 55.0 ± 17.4 | 65.3 ± 15.5 | 58.7 ± 20.3b |
Data are expressed as mean ± standard deviation. Comparisons among treatment groups were tested by using analysis of variance.
KD, kidney disease; NMES, neuromuscular electrical stimulation; NMES + P, neuromuscular electrical stimulation combined with protein supplementation; P, protein supplementation; RE, role limitations due to emotional problems; RP, role limitations due to physical health problem.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 434 View
- 18 Download
- ORCID iDs
-
Jae Hyeon Park

https://orcid.org/0000-0001-5619-4818Jae Yoon Park

https://orcid.org/0000-0001-8986-7492Geum Sil Lee

https://orcid.org/0000-0001-5449-1288Ran-hui Cha

https://orcid.org/0000-0003-2783-2600 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















