| Kidney Res Clin Pract > Volume 39(3); 2020 > Article |
|
Abstract
Background
Methods
Results
Conclusion
Acknowledgments
Notes
Funding
This research was supported by the Hallym University Research Fund in 2016 (HURF-2016-11) and an Inha University Research Grant (INHA-53356).
AuthorsŌĆÖ contributions
Yo Han Ahn, Juyoung Lee, and Tae-Jung Sung conceived and designed the study. Yo Han Ahn, Juyoung Lee, and Jiyoung Chun participated in data collection. Yo Han Ahn, Juyoung Lee, Jiyoung Chun, Yong Hoon Jun, and Tae-Jung Sung performed the study. Yo Han Ahn and Juyoung Lee performed statistical analysis and interpretation of data. Yo Han Ahn, Juyoung Lee, and Tae-Jung Sung wrote the manuscript. Tae-Jung Sung and Yong Hoon Jun revised the manuscript. All authors read and approved the final manuscript.
Figure┬Ā2
Differences in serum creatinine (S-Cr) and urine biomarkers levels between infants without acute kidney injury (AKI) and with AKI of gestational age < 28 weeks.

Figure┬Ā3
Differences in serum creatinine (S-Cr) and urine biomarker levels between infants without acute kidney injury (AKI) and with AKI of gestational age 28 to < 32 weeks.

Figure┬Ā4
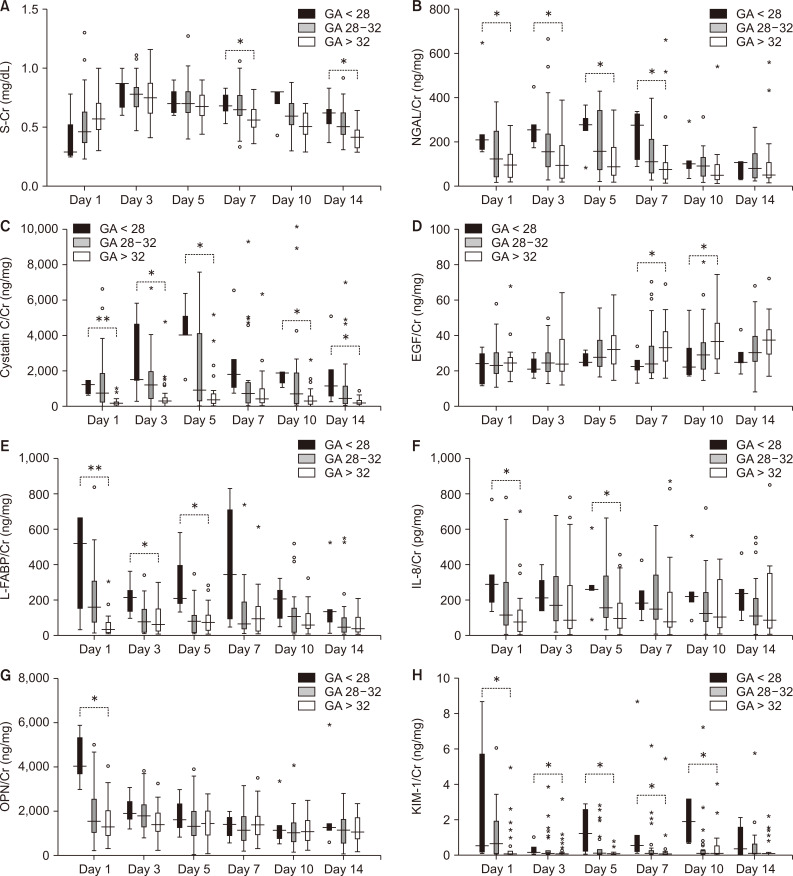
Differences in serum creatinine (S-Cr) and urine biomarkers levels according to gestational age (GA) in infants without acute kidney injury.
Table┬Ā1
| Characteristic | GA 32 to < 37 weeks (n = 30) | GA 28 to < 32 weeks (n = 35) | GA < 28 weeks (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| No AKI (n = 31) | AKI (n = 4) | P value | Adjusted P valuea | No AKI (n = 5) | AKI (n = 13) | P value | Adjusted P valuea | |||
| Maternal data | ||||||||||
| Age (yr) | 33.0 (32.0 to 35.0) | 35.0 (32.0 to 36.5) | 32.5 (30.0 to 34.5) | 0.352 | - | 35.0 (34.0 to 41.0) | 31.5 (29.5 to 34.0) | 0.064 | 0.998 | |
| Preeclampsia | 2 (6.7) | 7 (22.6) | 1 (25.0) | 1.000 | - | 0 (0.0) | 0 (0.0) | 1.000 | - | |
| Gestational diabetes | 4 (13.3) | 4 (12.9) | 0 | 1.000 | - | 4 (80.0) | 0 (0.0) | 0.002 | 0.996 | |
| Histological CAM | 4/22 (18.2) | 8/25 (32.0) | 1/4 (25.0) | 1.000 | - | 3/5 (60.0) | 3/13 (23.1) | 0.268 | - | |
| Placental infarction | 4/22 (18.2) | 5/25 (20.0) | 2/4 (50.0) | 0.238 | - | 0/5 (0.0) | 1/13 (7.7) | 1.000 | - | |
| Serum Cr (mg/dL) | 0.50 (0.43 to 0.60) | 0.50 (0.47 to 0.60) | 0.52 (0.50 to 0.55) | 0.352 | - | 0.42 (0.42 to 0.48) | 0.48 (0.40 to 0.53) | 0.442 | - | |
| Infant data | ||||||||||
| Sex, male:female | 17:13 | 17:14 | 2:2 | 1.000 | - | 4:1 | 8:5 | 0.615 | - | |
| GA (wk) | 33.6 (33.0 to 33.9) | 30.4 (29.1 to 30.9) | 29.0 (28.3 to 29.6) | 0.064 | 0.101 | 27.0 (26.7 to 27.1) | 26.0 (25.1 to 26.0) | 0.033 | 0.996 | |
| Weight (g) | 1,980 (1,740 to 2,320) | 1,380 (1,245 to 1,530) | 1,130 (1,105 to 1,190) | 0.020 | 0.108 | 990 (840 to 1,010) | 840 (730 to 950) | 0.200 | - | |
| Weight, Z score | -0.19 (-0.60 to 0.42) | 0.34 (-0.37 to 0.65) | -0.05 (-0.51 to 0.34) | 0.499 | - | 0.20 (-0.10 to 0.22) | 0.19 (-0.07 to 0.69) | 0.805 | - | |
| Height, cm | 45.3 (43.5 to 48.0) | 40.0 (39.3 to 42.6) | 38.5 (37.5 to 39.5) | 0.082 | 0.937 | 37.0 (33.5 to 37.0) | 36.0 (33.5 to 37.0) | 0.653 | - | |
| Height, Z score | 0.44 (-0.16 to 1.51) | 0.90 (0.21 to 1.65) | 0.83 (0.08 to 0.87) | 0.467 | - | 0.82 (-0.51 to 0.83) | 1.23 (-0.15 to 1.61) | 0.349 | - | |
| Small for GA | 3 (10.0) | 3 (9.7) | 0 (0.0) | 1.000 | - | 0 (0.0) | 0 (0.0) | 1.000 | - | |
| Apgar score, 1 min | 7.0 (6.0 to 7.0) | 5.0 (3.0 to 6.0) | 3.5 (2.5 to 5.0) | 0.379 | - | 2.0 (2.0 to 3.0) | 2.0 (1.0 to 4.0) | 0.775 | - | |
| Apgar score, 5 min | 8.0 (7.0 to 9.0) | 7.0 (6.0 to 8.0) | 5.0 (4.5 to 6.0) | 0.144 | 0.595 | 5.0 (4.0 to 6.0) | 5.0 (4.0 to 6.0) | 0.633 | - | |
| CRIB II score | - | 5.0 (4.0 to 7.0) | 7.5 (5.5 to 8.0) | 0.104 | 0.558 | 9.0 (7.5 to 10.0) | 11.0 (10.0 to 12.0) | 0.026 | 0.996 | |
| Antenatal steroid | 20/28 (71.4) | 26/29 (89.7) | 4 (100.0) | 1.000 | - | 5 (100.0) | 13 (100.0) | 1.000 | - | |
| RDS | 4 (13.3) | 20 (64.5) | 2 (50.0) | 0.618 | - | 5 (100.0) | 12 (92.3) | 1.000 | - | |
| Treatment for PDA | 1 (3.3) | 2 (6.5) | 1 (25.0) | 0.313 | - | 1 (20.0) | 9 (69.2) | 0.118 | 0.999 | |
| Proven sepsis | 0 (0.0) | 7 (22.6) | 1 (25.0) | 1.000 | - | 1 (20.0) | 3 (23.1) | 1.000 | - | |
| NEC Ōēź stage 2 | 1 (3.3) | 4 (12.9) | 2 (50.0) | 0.128 | 0.447 | 0 (0.0) | 2 (15.4) | 1.000 | - | |
| Hospital stay (d) | 18 (14 to 25) | 47 (42 to 57) | 82 (70 to 107) | 0.001 | < 0.001 | 91 (79 to 99) | 96 (85 to 105) | 0.775 | - | |
Table┬Ā2
| GA 28 to < 32 weeks (n = 4) | GA < 28 weeks (n = 13) | P value | |
|---|---|---|---|
| Onset of AKI (d) | 7 (5-10) | 7 (7-9) | 0.404 |
| Cause of AKI | 0.053 | ||
| Hemodynamically significant PDA | 1 (25.0) | 7 (53.8) | |
| Sepsis | 0 (0.0) | 2 (15.4) | |
| Drug-induced | 0 (0.0) | 3 (23.1) | |
| NEC | 2 (50.0) | 0 (0.0) | |
| Unknown | 1 (25.0)a | 1 (7.7)b | |
| AKI stage | 0.643 | ||
| Stage 1 | 3 (75.0) | 7 (53.8) | |
| Stage 2 | 1 (25.0) | 4 (30.8) | |
| Stage 3 | 0 (0.0) | 2 (15.4) | |
| Kidney replacement therapy | 0 (0.0) | 0 (0.0) | 1.000 |
| Mortality | 0 (0.0) | 1 (7.7) | 1.000 |
| Renal sequelae at discharge | |||
| eGFR, mL/min/1.73 m2 | 71.6 (67.3-107.5) | 101.1 (57.4-109.0) | 0.910 |
| Reduced eGFRc | 0 (0.0) | 0 (0.0) | 1.000 |
| Proteinuria | 2 (50.0) | 2 (15.4) | 0.219 |
| Abnormal findings on USG | 0 (0.0) | 0 (0.0) | 1.000 |
AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; GA, gestational age; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; USG, ultrasonography.
aThis patient had histological chorioamnionitis, and Ureaplasma urealyticum was identified from the first gastric juice, which was regarded as amniotic fluid. The patient also showed a leukemoid reaction for the first 2 weeks of life (white blood cell, 57,000-112,800/┬ĄL) without any infection focus, which resolved spontaneously. bThis patient had no specific cause related to AKI, except increased maternal level of C-reactive protein before birth. ceGFR < 30, < 49, and < 72 mL/min/1.73 m2 at < 42 weeks, 42 to < 48 weeks, and Ōēź 48 weeks of postmenstrual age, respectively.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















