Effects of Toll-like receptor antagonist 4,5-dihydro-3-phenyl-5-isoxasole acetic acid on the progression of kidney disease in mice on a high-fat diet
Article information
Abstract
Background
Obesity-related metabolic disorders are closely associated with inflammation induced by innate immunity. Toll-like receptors (TLRs) play a pivotal role in the innate immune system by activating proinflammatory signaling pathways. GIT27 (4,5-dihydro-3-phenyl-5-isoxasole acetic acid) is an active immunomodulatory agent that primarily targets macrophages and inhibits secretion of tumor necrosis factor α [as well as interleukin (IL)-1β, IL-10, and interferon γ]. However, the effect of TLR antagonist on kidney diseases has rarely been reported. We investigated whether the TLR antagonist GIT27 has beneficial effects on the progression of kidney disease in obese mice on a high-fat diet (HFD).
Methods
Six-week-old male C57BL/6 mice were divided into three groups: mice fed with normal chow diet (N=4); mice fed with a HFD (60% of total calories from fat, 5.5% from soybean oil, and 54.5% from lard, N=4); and GIT27-treated mice fed with a HFD (N=7).
Results
Glucose intolerance, oxidative stress, and lipid abnormalities in HFD mice were improved by GIT27 treatment. In addition, GIT27 treatment decreased the urinary excretion of albumin and protein in obesity-related kidney disease, urinary oxidative stress markers, and inflammatory cytokine levels. This treatment inhibited the expression of proinflammatory cytokines in the kidneys and adipose tissue, and improved extracellular matrix expansion and tubulointerstitial fibrosis in obesity-related kidney disease.
Conclusion
TLR inhibition by administering GIT27 improved metabolic parameters. GIT27 ameliorates abnormalities of lipid metabolism and may have renoprotective effects on obesity-related kidney disease through its anti-inflammatory properties.
Introduction
Obesity, insulin resistance, type 2 diabetes, dyslipidemia, and hypertension together comprise metabolic syndrome [1]. Obesity is also the most important risk factor for hypertension, metabolic disease, diabetes mellitus, and chronic renal disease. For a long time, the kidneys have been considered a victim of metabolic syndrome, and abnormalities of lipid and glucose metabolism in the kidneys are as important as those in adipose tissue. Therefore, the possibility of cross-talk between adipose tissue and the kidneys exists in metabolic syndrome, particularly in chronic renal diseases related to insulin resistance such as obesity and diabetes mellitus [2]. Injuries in the structures of the kidney, leading to changes in renal structure including glomerular hypertrophy, mesangial expansion, abnormalities of the podocyte foot processes, increased glomerular capillary wall tension, and vascular abnormalities, are observed in obese humans and animals. These changes decrease the glomerular filtration rate and increase proteinuria or albuminuria [1], [3], [4].
Recent research suggests that obesity-related metabolic disorders are closely associated with inflammation induced by innate immunity. The adipose tissue contains adipocytes, stromal-vascular cells, endothelial cells, fibroblasts, and macrophages. Obesity induces an increased infiltration of activated macrophages into the adipose tissue. Macrophages contribute to the increased secretion of proinflammatory cytokines such as tumor necrosis factor α (TNFα), monocyte chemoattractant protein-1 (MCP-1), interleukin (IL)-6, C-reactive protein (CRP), and plasminogen activator inhibitor-1 (PAI-1) in the adipose tissue. Obesity also induces an increase in plasma free fatty acid (FFA) concentration due to the supersaturation of fat storage in the adipose tissue. Increased levels of proinflammatory cytokines and FFAs induce systemic inflammation, oxidative stress, and insulin resistance through the activation of immune receptors and stress signaling pathways [5], [6], [7], [8].
Toll-like receptors (TLRs) belong to a family of pattern-recognition receptors that play a pivotal role in the innate immune system by activating proinflammatory signaling that mainly helps in recognizing specific pathogen-associated molecular patterns. These patterns include exogenous patterns such as lipopolysaccharide (LPS), mycoplasma lipoproteins, viral and bacterial nucleic acids, endogenous ligands such as heat shock protein (HSP) 60 and HSP70, saturated fatty acids, unsaturated fatty acids, hyaluronic acid fragment, and surfactant protein-A. An increased plasma FFA level in obesity induces inflammation through the TLR4 signaling pathway [9], [10], [11], [12]. In previous studies, TLR has been known to be associated with multiple renal dysfunctions, including lupus nephritis, antibody-mediated glomerulonephritis, acute allograft rejection, endotoxin-induced acute renal failure, kidney ischemia/reperfusion injury, and diabetic kidney disease [13], [14], [15], [16], [17], [18].
GIT27 (4,5-dihydro-3-phenyl-5-isoxasole acetic acid) is an active immunomodulatory agent that primarily targets macrophages and inhibits the secretion of LPS-induced TNFα [as well as IL-1β, IL-10, and interferon (IFN)-γ] via interference of the macrophage TLR4 and TLR2/6 signaling pathways. In addition to inhibiting proinflammatory cytokine synthesis, GIT27 inhibits LPS-induced nuclear factor kappa B (NFκB) and p38 mitogen-activated protein (MAP) kinase signaling pathways. GIT27 has showed therapeutic effects in multiple inflammatory diseases, including type 1 diabetes mellitus, rheumatoid arthritis, and colitis [19], [20], [21]. In this study, we observed that an immunonodulator such as GIT27 has beneficial effects on obesity-related metabolic parameters, renal injury, and lipid metabolism in both the kidneys and the adipose tissue.
Methods
Animal experiments
C57BL/6 mice were caged individually under controlled temperature (23±2°C) and humidity (55±5%) with an artificial light cycle. Six-week-old male C57BL/6 mice were divided into three groups: mice fed with a normal chow diet (ND, N=4); mice fed with a high-fat chow diet (HFD, 60% of total calories from fat, 5.5% from soybean oil, and 54.5% from lard; N=4); and GIT27-treated mice fed with a HFD (N=7). GIT27 was purchased from Tocris Bioscience (Tocris Cookson, Ellisville, MI, USA) and administered via an intraperitoneal route at a dose of 20 mg/kg daily for 12 weeks. We measured the food and water intake, urine volume, body weight, fasting blood glucose concentration, and HbA1c level every month. Plasma glucose level was measured using the glucose oxidase method, and HbA1c level was calculated using the IN2IT system (Bio-Rad Laboratories, Hercules, CA, USA). Plasma creatinine level was determined using high-performance liquid chromatography. Plasma insulin and adiponectin levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Linco Research, St Charles, MO, USA). The homeostasis model assessment index (HOMA-IR) was calculated using the following formula: [fasting glucose (mmol/L)×fasting insulin (mU/L)/22.5]. Plasma triglyceride and cholesterol analyses were performed using a GPO-Trinder kit (Sigma, St Louis, MO, USA). For the glucose tolerance test, we performed oral gavage of 3 g dextrose/kg in mice after a 5-hour fast and collected blood samples through the tail vein. Individual mice were separated in a metabolic cage where urine was collected and measured for 24 hours every month to determine the amount of urinary albumin excretion by competitive ELISA (Shibayagi, Shibukawa, Japan), which was corrected by the urinary creatinine concentration. The urinary level of 8-isoprostane was measured using an ELISA kit (Cayman Chemical, Ann Arbor, MI, USA). The levels of proinflammatory cytokines (IFN-γ and IL-2) in urine were measured using the Millipore Milliplex mouse cytokine/chemokine kit. At the end of the study period, systolic blood pressure was measured using tail-cuff plethysmography (LE 5001-Pressure Meter; Letica SA, Barcelona, Spain). Mice were sacrificed under anesthesia with intraperitoneal injections of sodium pentobarbital (50 mg/kg). Their heart, epididymal fat, liver, and kidney tissues were extracted and subsequently snap-frozen in liquid nitrogen. Lipids from the hepatic, adipose, and renal cortical tissues were extracted using the Bligh and Dyer method [22]. Total cholesterol and triglyceride contents were measured using a commercial kit (Wako Chemicals, Richmond, VA, USA). Plasma and tissue FFA levels were also measured using a commercial kit (Abcam Plc, Cambridge, MA, USA). The extent of peroxidative reaction in the hepatic tissue, adipose tissue, and kidneys was determined by direct measurement of lipid hydroperoxides (LPO) using an LPO assay kit (Cayman Chemical), as described previously [23]. We performed all the experiments in accordance with the guidelines of the National Institutes of Health and with the approval of the Korea University Institutional Animal Care and Use Committee.
Light microscopy and immunohistochemistry
We fixed kidney tissues in 4% paraformaldehyde and embedded them in paraffin. They were then cut into 4-μm-thick slices and stained with periodic acid–Schiff. Glomerular mesangial expansion was scored semiquantitatively, and the percentage of mesangial matrix occupying each glomerulus was rated from 0 to 4 as follows: 0, 0%; 1, <25%; 2, 25–50%; 3, 50–75%; and 4, >75%. After the removal of paraffin, the slices were dehydrated in xylene followed by in graded alcohol and immersed in distilled water. The free kidney sections were transferred to 10 mmol/L citrate buffer adjusted to a pH of 6.0 for various staining methods. Sections were microwaved for 10–20 minutes to retrieve the antigens for transforming growth factor β 1 (TGFβ1) and F4/80, and transferred to Biogenex Retrievit (pH 8.0) (InnoGenex, San Ramon, CA, USA) for PAI-1 staining or trypsin treatment (Sigma) for 30 minutes at 37°C, to detect type IV collagen. For blocking the endogenous peroxidase activity, 3.0% H2O2 in methanol was applied to the tissues for 20 minutes, followed by incubation at room temperature for 60 minutes with 3% bovine serum albumin (BSA)/3% normal goat serum (type IV collagen), 90 minutes with 5% normal goat serum, and either 15 minutes with 10% powerblock (PAI-1) or 30 minutes with 20% normal sheep serum (TGFβ1). Slides were incubated overnight at 4°C with rabbit polyclonal anti-TGFβ1 antibody (1:200; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), mouse monoclonal anti-F4/80 antibody (1:100; Serotec, Oxford, UK), rabbit polyclonal anti-type IV collagen antibody (1:200; Santa Cruz Biotechnology Inc.), or rabbit polyclonal anti-PAI-1 antibody (1:50; American Diagnostica, Stanford, CT, USA). After overnight incubation, the slides were incubated again with a secondary antibody for 30 minutes and detected by incubation at room temperature with a mixture of 0.05% 3,3′-diaminobenzidine containing 0.01% H2O2 and counterstaining with Mayer’s hematoxylin. Negative control sections were stained under identical conditions, but with a buffer solution instead of the primary antibody. For the evaluation of immunohistochemical staining for type IV collagen, TGFβ1, PAI-1, and F4/80, the glomerular fields were graded semiquantitatively under a high-power field containing 50–60 glomeruli, and the average score was calculated as described previously [23]. A pathologist examined and scored the histological changes in a blinded manner.
Analysis of gene expression by real-time quantitative polymerase chain reaction
We extracted total RNA from the experimental cells using the Trizol reagent. Primers were designed for their respective gene sequences using the Primer 3 software, and the secondary structures of the templates were examined and excluded using the mfold program. Quantitative gene expression was performed using a LightCycler 1.5 system (Roche Diagnostics Corporation, Indianapolis, IN, USA) using SYBR Green technology. In 96-well real-time polymerase chain reaction (PCR) plates, 10 μL SYBR Green master mix was added to 1 μL of RNA (corresponding to 50 ng of total RNA) and 900nM of both forward and reverse primers for a total reaction volume of 20 μL. Real-time reverse transcriptase PCR was performed for 10 minutes at 50°C and for 5 minutes at 95°C. Thirty PCR cycles of denaturation for 10 seconds at 95°C and annealing with extension for 30 seconds at 60°C were then conducted. Gene-level ratios relative to β-actin (relative gene expression number) were calculated by subtracting the threshold cycle number of the target gene from that of β-actin and squaring this difference. We evaluated the specificity of each PCR product using melting curve analysis, followed by agarose gel electrophoresis.
Protein extraction and Western blot analysis
We extracted nuclear and cytoplasmic proteins from renal cortical tissues and cells using a commercial nuclear extraction kit according to the manufacturer’s instructions (Active Motif, Carlsbad, CA, USA). Protein concentration was determined using the bicinchoninic acid method (Pierce Pharmaceuticals, Rockford, IL, USA). For Western blotting, 40 μg of protein was electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) mini-gel. Proteins were transferred onto a polyvinylidene difluoride membrane and the membrane was hybridized in blocking buffer overnight at 4°C with goat polyclonal anti-TLR4 antibody (1:500, Santa Cruz Biotechnology Inc.), goat polyclonal anti-TRAF6 antibody (1:500, Santa Cruz Biotechnology Inc.), rabbit polyclonal anti-NOX4 antibody (1:500, Novus Biologicals), rabbit polyclonal anti-NFκB (p65) antibody (1:1,000, Cell Signaling Technology, Danvers, MA, USA), mouse monoclonal anti-inhibitory kappa B (IκB)β antibody (1:1,000, Cell Signaling Technology), anti-p-AMP-dependent kinase (AMPK) antibody, anti-AMPK antibody, anti-sterol regulatory element binding transcription protein1 (SREBP1) antibody, and mouse monoclonal anti-β-actin antibody (1:5,000, Sigma). The membrane was subsequently incubated with horseradish peroxidase-conjugated secondary antibody (diluted 1:1,000) for 60 minutes at room temperature. We detected the specific signals using an enhanced chemiluminescence method (Amersham, Buckinghamshire, UK).
Cell culture
We cultured podocytes to elucidate the molecular mechanism of TLR4 because TLR4 expression was detected preferentially in glomerular podocytes. Conditionally immortalized mouse podocytes were cultured as described previously [24]. Briefly, frozen podocytes were first grown under permissive conditions at 33°C in RPMI 1640 media containing 10% fetal bovine serum (FBS), 50 U/mL IFN-γ, and 100 μg/mL antibiotics in collagen-coated flasks. The IFN-γ was gradually reduced to 10 U/mL in successive passages. Cells were then trypsinized and subcultured without IFN-γ (nonpermissive conditions) and allowed to differentiate at 37°C, with changing the medium on alternate days. Differentiation of the podocytes grown for at least 8 days at 37°C was confirmed by identification of the podocyte differentiation marker synaptopodin using Western blotting (data not shown). The 3T3-L1 cells were obtained from American Type Culture Collection (Manassas, VA, USA) and were maintained in Dulbecco’s modified Eagle’s medium containing 10% FBS and 100 μg/mL antibiotics at 37°C under a humidified 5% CO2 atmosphere. Differentiation of the preadipocytes into adipocytes was induced by the addition of a hormone cocktail, as described previously [25]. Briefly, 2 days after confluence, the medium was replaced with a standard adipocyte differentiation induction medium containing 0.5 mmol/L isobutylmethylxanthine, 1 μmol/L dexamethasone, and 10 μg/mL insulin. Identification of oil-red O-stained lipid droplets in the cytoplasm, quantification of the intracytoplasmic lipids, and the expression of peroxisome proliferator activator receptorγ (PPARγ) (data not shown) were used to confirm the differentiation of adipocytes. For palmitate preparation, palmitate-free acids and low-endotoxin BSA (FFA free) were purchased from Sigma-Aldrich. A 20mM solution of palmitate was dissolved in 0.01M NaOH, incubated at 70°C for 30 minutes, and complexed to 10% BSA at a molar ratio of 6.6:1 in podocytes and 3:1 in adipocytes. The solution was shaken overnight at 37°C under N2, sonicated for 10 minutes, filter sterilized, and stored at 20 °C prior to use [26], [27]. Cells were cultivated on 100-mm dishes and serum restricted for 24 hours. These were subsequently exposed to normal glucose (NG; 5.5mM) or high glucose (HG; 30mM) with or without palmitate (100μM) for 24 hours. To evaluate the effects of TLR antagonism, GIT27 was added to the cells 2 hours prior to exposure at a concentration of 10 μg/mL. After the experiment, the cell lysates and conditioned media were centrifuged at 15,000 rpm for 10 minutes at 4°C, and the supernatants were collected. All experimental groups were cultured in triplicate and harvested at 24 hours.
Statistical analysis
We used nonparametric analysis for the relatively small number of samples. Results are expressed as mean±standard error of the mean. Comparisons were made using the Wilcoxon rank sum test and Bonferroni correction. A Kruskal–Wallis test was used for comparison of more than two groups, followed by a Mann–Whitney U test. A P value of <0.05 was considered statistically significant. The statistical analyses were performed using SPSS for Windows, version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Biochemical parameters in the experimental animals
The physical and biochemical parameters for each group of the experimental animals are shown in Table 1. As expected, body weights of HFD mice were much higher than those of ND mice, despite the former group taking smaller amounts of food and water. However, interestingly, GIT27 treatment reduced body weight in HFD mice without any changes in food and water intake. We observed significant changes in fat content of each organ and in kidney weight. Fat weight increased significantly in HFD mice and decreased with GIT27 treatment. However, kidney weight decreased in HFD mice and was restored with GIT27 treatment. No changes were observed in the weight of the heart and liver. In HFD mice, larger urinary volumes were recorded, which were not affected by GIT27. HFD mice did not show any significant changes in systolic blood pressure and serum creatinine level as compared to ND mice. The plasma glucose and HbA1c levels were not different among the three groups. The plasma insulin level was higher in HFD mice than in ND mice, but this difference was not significant. Among the three groups, no changes were observed in plasma adiponectin. HOMA-IR was significantly lower in HFD mice treated with GIT27 for 8 weeks compared to those without GIT27 treatment.
Metabolic effects, lipid peroxidation, and oxidative stress of GIT27 in experimental animals
To evaluate glucose tolerance, we measured blood glucose levels after oral dextrose gavage every 4 weeks. Interestingly, GIT27 treatment improved blood glucose levels in HFD mice significantly at 4, 8, and 12 weeks, although variable levels were observed in ND mice after 12 weeks (Fig. 1A–D). In accordance with glucose tolerance, lipid abnormalities also improved significantly in HFD mice by GIT27 treatment (Fig. 2). In addition, lipid contents in the kidneys, fat, and liver also showed a significant decrease by GIT27 treatment (Fig. 3A and B). We also compared the oxidative stress in each organ among the three groups. An assessment of the LPO contents and urinary 8-isoprostane levels of the kidneys, fat, and liver showed that the LPO contents were markedly higher in HFD mice, which were decreased significantly by GIT27 treatment (Fig. 3C). The level of urinary 8-isoprostane was increased markedly in HFD mice and decreased in GIT27-treated HDF mice, but the differences were not significant (Fig. 3D).
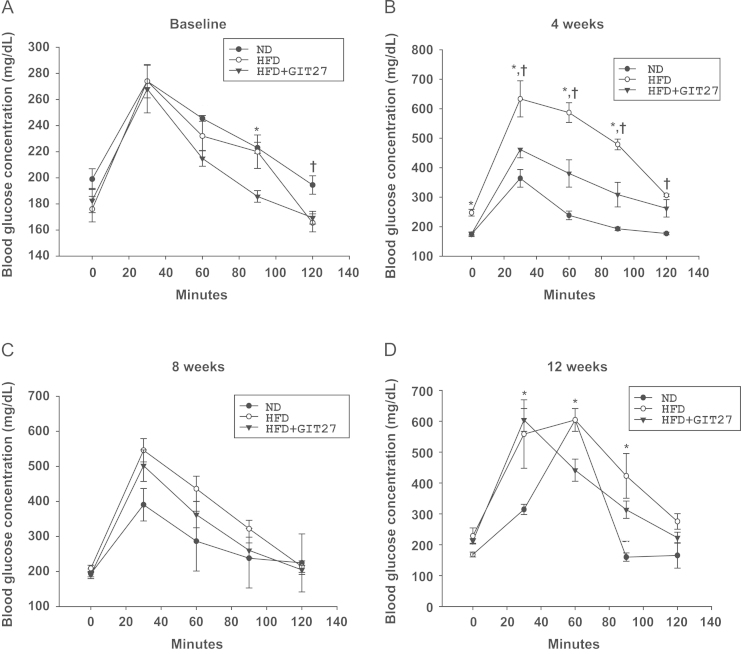
Effectsof GIT27 on glucose tolerance test. After 4 weeks, plasma glucose levels increased significantly in HFD mice compared with those in ND mice (P<0.05). GIT27 treatment improved glucose tolerance (P<0.05). * P<0.05, HFD versus HFD+GIT27. †P<0.05, ND versus HFD. GIT27, 4,5-dihydro-3-phenyl-5-isoxasole acetic acid; HFD, high-fat chow diet; ND, normal chow diet.
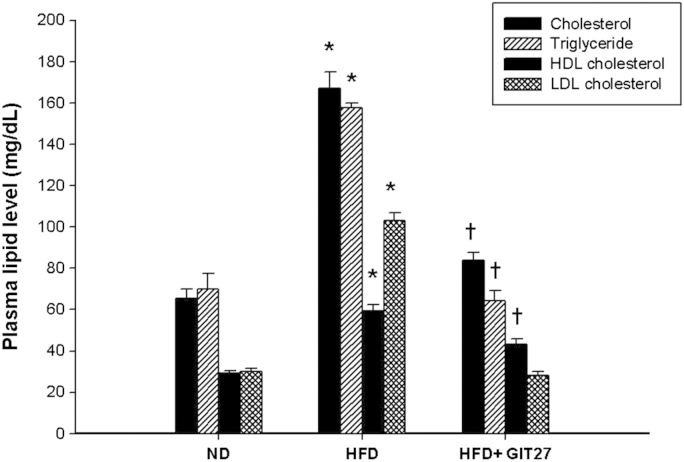
Effects of GIT27 on plasma lipid profiles. Lipid abnormalities improved significantly in GIT27-treated HFD mice (P<0.05). * P<0.05, ND versus HFD. †P<0.05, HFD versus HFD+GIT27. GIT27, 4,5-dihydro-3-phenyl-5-isoxasole acetic acid; HDL, high-density lipoprotein; HFD, high-fat chow diet; LDL, low-density lipoprotein; ND, normal chow diet.
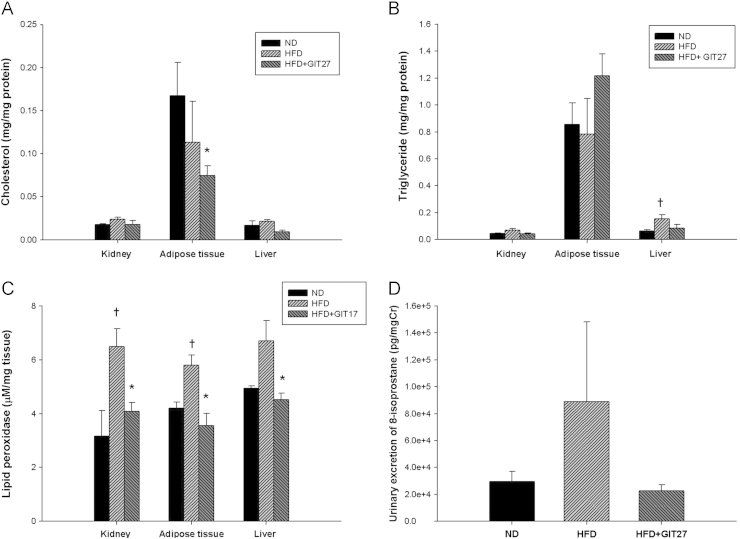
Effects of GIT27 on tissue lipid content, lipid peroxidation, and oxidative stress. Compared to the kidneys and liver, the level of (A) cholesterol and (B) triglyceride were higher in the adipose tissue. (C) Lipid peroxide levels in the kidneys, adipose tissue, and liver were markedly higher in HFD mice and decreased significantly in GIT27-treated HFD mice. (D) Urinary 8-isoprostane was increased markedly in HFD mice and decreased by GIT27 treatment. * P<0.05, ND versus HFD. †P<0.05, ND versus HFD. Cr, creatinine; GIT27, 4,5-dihydro-3-phenyl-5-isoxasole acetic acid; HFD, high-fat chow diet; ND, normal chow diet.
Renal effects of GIT27 in experimental animals
To examine the effect of GIT27 on renal injury, we first measured urinary excretion of protein and albumin. Urinary protein excretion was increased markedly in HFD mice compared with that in ND mice, as shown in Fig. 4A. However, urinary protein excretion was improved significantly after treatment with GIT27. Fig. 4B shows the urine albumin excretion in 24 hours. After 12 weeks, urinary albumin excretion was also improved significantly in GIT27-treated HFD mice compared with HFD mice without treatment. Next, we evaluated the anti-inflammatory effect of GIT27 on obesity-induced renal injury after confirming metabolic improvement and an antiproteinuric effect. We also compared the urinary levels of proinflammatory cytokines (TNFα and IL-2) among the three groups based on an analysis of the 24-hour urine samples using an ELISA kit. The levels of TNFα and IL-2 in the urine were increased in HFD mice and decreased in GIT27-treated HFD mice. In particular, the IL-2 level was improved significantly in GIT27-treated HFD mice compared with HFD mice without treatment (Fig. 5A). Moreover, we evaluated the gene expression of the adipose and kidney tissues to confirm the anti-inflammatory and antifibrotic effects of GIT27 treatment. The gene expression of proinflammatory cytokines such as MCP-1, PAI-1, and TNFα in the adipose tissue was increased in HFD mice and decreased by GIT27 treatment (Fig. 5B). No significant change was recorded in PPARγ and adiponectin gene expression. AMPK gene expression was increased significantly in HFD mice, which decreased by GIT27 treatment. Gene expressions of proinflammatory and profibrotic cytokines in the kidney tissue tended to increase in HFD mice and decrease in those treated with GIT27. In particular, gene expressions of type IV collagen, PAI-1, MCP-1, and IL-1b were decreased significantly in GIT27-treated HFD mice compared with HDF mice without treatment (Fig. 5B and C). With regard to lipid metabolism in the kidney tissue, SREBP1c gene expression was reduced by GIT27 treatment.
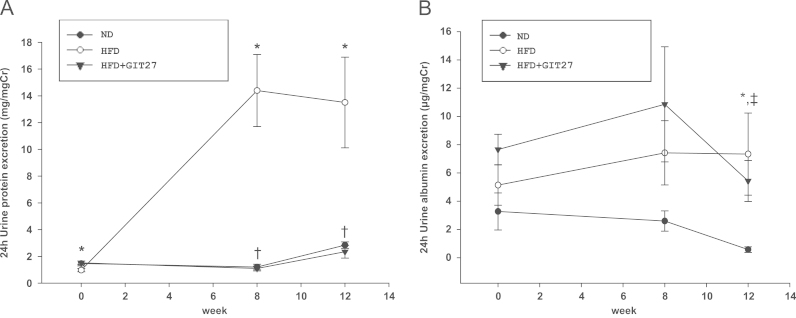
Effects of GIT27 on proteinuria. Urinary protein excretion was significantly higher in HFD mice than in ND mice. (A) This was improved significantly after GIT27 treatment. (B) After 12 weeks of experiment, urinary albumin excretion was improved markedly in GIT27-treated HFD mice compared with that in HFD mice. * P<0.05, ND versus HFD. †P<0.05, HFD versus HFD+GIT27. ‡P<0.05, ND versus HFD+GIT27. Cr, creatinine; GIT27, 4,5-dihydro-3-phenyl-5-isoxasole acetic acid; HFD, high-fat chow diet; ND, normal chow diet.

Anti-inflammatory and antifibrotic effects of GIT27. (A) Levels of TNFα and IL-2 in urine were increased in HFD mice and decreased in GIT27-treated HFD mice. In particular, the level of IL-2 was improved significantly in GIT27-treated HDF mice compared with that in HFD mice. (B) The gene expression of proinflammatory cytokines such as MCP-1, PAI-1, and TNFα in the adipose tissue tended to increase in HFD mice and decrease in GIT27-treated HFD mice. (C and D) The gene expression of proinflammatory and profibrotic cytokines in kidney tissue tended to increase in HFD mice and decrease in GIT27-treated HFD mice and that of type IV collagen, PAI-1, MCP-1, IL-1b, and SREBP1 were decreased significantly in GIT27-treated HFD mice compared with that in HFD mice. * P<0.05, HFD versus HFD+GIT27. †P <0.05, HFD versus HFD+GIT27. ‡P<0.05, ND versus HFD. ABCA, adenosine triphosphate-binding cassette transporter A; AMPK, AMP-dependent kinase; Col(IV), type lV collagen alpha; Cr, creatinine; FAS, fatty acid synthase; GIT27, 4,5-dihydro-3-phenyl-5-isoxasole acetic acid; HFD, high-fat chow diet; HMG coA, 3-hydroxy-3-methylglutaryl-coenzyme A; IL, interleukine; INF-γ, interferon gamma; MCP, monocyte chemoattractant protein; ND, normal chow diet; PAI, plasminogen activator inhibitor; PPARγ, peroxisome proliferator activator receptor gamma; SREBP, sterol-regulatory element-binding protein; TGF, transforming growth factor; TNF, tumor necrosis factor.
Fig. 6 shows the representative immunohistochemical findings for each group of experimental animals. We observed higher extracellular matrix expansion and tubulointerstitial fibrosis, and increased expression of type IV collagen, TGFβ1, and PAI-1 in HFD mice. Consistent with the results of the urinary albumin excretion, the expression of these genes was decreased by GIT27 treatment compared to that in HFD mice without treatment. The expression of F4/80 as a macrophage marker was increased in HFD mice and reduced in GIT27-treated HFD mice. This result suggests that GIT27 restored the renal inflammatory changes by suppressing macrophage accumulation in the kidneys.
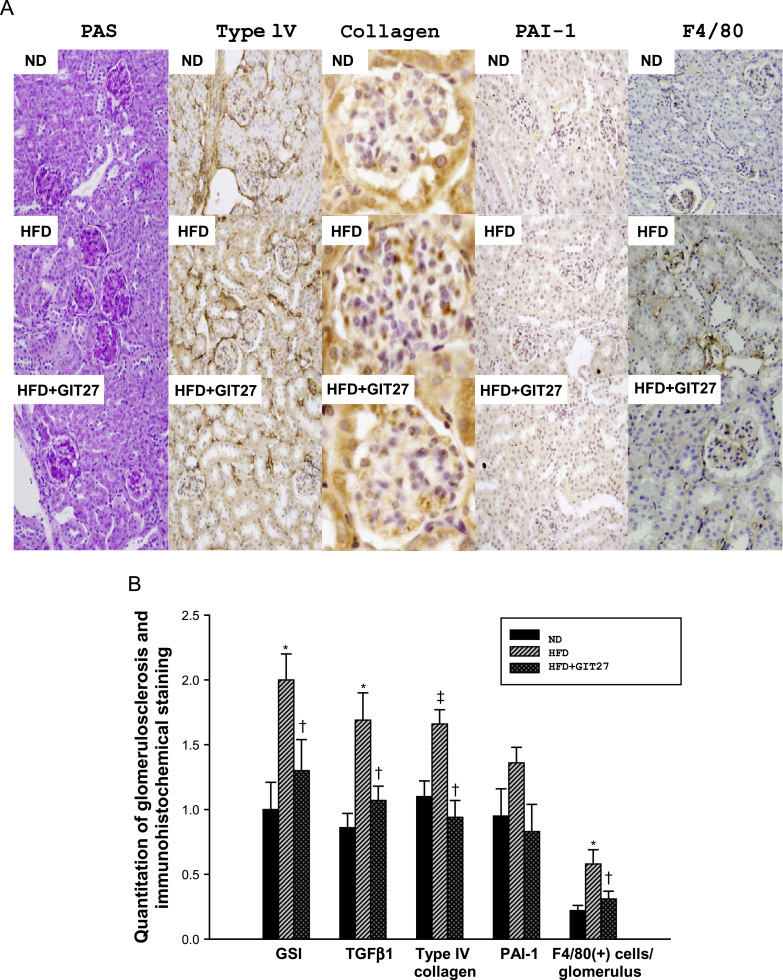
Renal pathology and immunohistochemistry. (A) Representative sections show that the histological changes and immunohistochemical staining for PAS, type IV collagen, PAI-1, F4/80, and TGFβ1 were increased in HFD mice and decreased in GIT27-treated HFD mice (original magnification 400× for PAS, type IV collagen, F4/80 staining, and TGF-β1 staining; and 1000× for PAI-1 staining). (B) Bar graphs represent quantification of glomerulosclerosis and the immunostaining scores in the kidney tissue. Data are shown as the mean±SEM. * P <0.01 ND versus HFD. †P<0.05 HFD versus HFD+GIT27. ‡P<0.05 ND versus HFD. GIT27, 4,5-dihydro-3-phenyl-5-isoxasole acetic acid; GSI, glomerulosclerosis index; HFD high-fat chow diet; ND, normal chow diet; PAI-1, plasminogen activator inhibitor-1; PAS, periodic acid-Schiff; SEM, standard error of the mean; TGF, transforming growth factor.
TLR4 pathway and the effect of GIT27 in experimental animals
To understand the mechanism of the beneficial effects of GIT27 relating to the TLR4 pathway, we investigated TLR4, NFκB, IκBβ, and NOX4 protein synthesis in the kidneys of ND mice and HFD mice with and without GIT27 treatment. The expression of TLR4 showed a tendency to increase in HFD mice, which decreased with GIT27 treatment; however, these changes were not significant. The expression of NFκB, IκBβ, and NOX4 also showed no change in HFD mice with and without GIT27 treatment (Fig. 7).
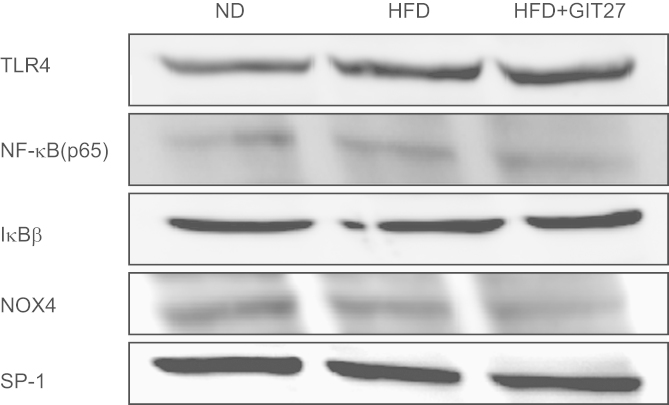
TLR4/NFκB pathway in animal experiments. Protein synthesis for TLR4, NFκB, IκBβ, and NOX4 did not show a significant change in renal cortical tissues among ND, HFD, and GIT27-treated HFD mice. GIT27, 4,5-dihydro-3-phenyl-5-isoxasole acetic acid; HFD, high-fat chow diet; IκB, inhibitory kappa B; ND, normal chow diet; NFκB, nuclear factor kappa B; NOX, nicotinamide adenine dinucleotide phosphate oxidase; TLR, Toll-like receptor.
TLR4 pathway in cultured podocytes and adipocytes under FFA conditions
We confirmed previously that TLR4 mRNA expression was increased markedly under FFA and HG conditions in cultured podocytes [18]. This effect was notable upon exposure to both HG and FFAs. Consistent with these changes, proinflammatory cytokine secretion into the culture supernatant was also increased tremendously in FFA conditions. Moreover, HG and FFA also increased NFκB activation. However, GIT27 interestingly inhibited TLR4, TNFα, NOX4 expression, NFκB activation, and proinflammatory cytokine synthesis. The protein synthesis results of this study agreed with the findings of previous studies. In particular, FFA induced NFκB activation and IκBβ inhibition in podocytes (Fig. 8). Additionally, GIT27 suppressed NFκB activation and reactivated IκBβ inhibition significantly. The protein expression of TLR4, TRAF6, NOX4, p-AMPK, and SREBP1 tended to increase under FFA conditions, but the trend was reversed by GIT27 treatment; however these changes were not significant in Western blot analysis. In addition, with regard to adipocytes, no significant changes were observed in the protein expression of SREBP1, TLR4, NFκB, IκBβ, NOX4, and p-AMPK (Fig. 9).
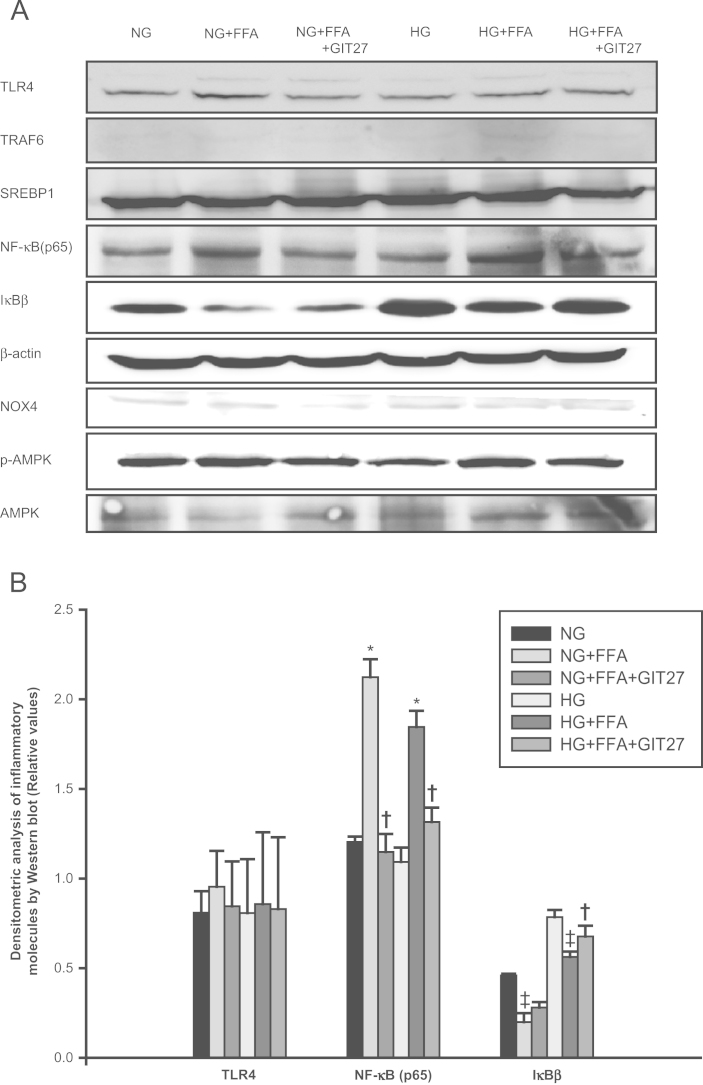
TLR4/NFκB pathway in cultured podocytes. (A, B) FFA induced NFκB activation and IκBβ inhibition in podocytes, which were suppressed by GIT27. TLR4, TRAF6, NOX4, p-AMPK, and SREBP1 protein expressions tended to increase after FFA treatment, an effect that was reversed by GIT27 treatment. However, these changes were not significant on densitometric analysis of Western blot results. Data are shown as mean±SEM. * P < 0.01 NG versus NG+FFA and HG versus HG+FFA. †P<0.05 NG+FFA versus NG+FFA+GIT27 and HG+FFA versus HG+FFA+GIT27. AMPK, AMP-dependent kinase; FFA, free fatty acid; GIT27, 4,5-dihydro-3-phenyl-5-isoxasole acetic acid; HG, high glucose; IκB, inhibitory kappa B; NFκB, nuclear factor kappa B; NG, normal glucose; NOX, nicotinamide adenine dinucleotide phosphate oxidase; SREBP1, sterol regulatory element binding transcription protein1; TLR, Toll-like receptor; TRAF, TNF receptor associated factor.

TLR4/NFκB pathway in adipocytes. In adipocytes, no changes were observed in the protein expression of SREBP1, TLR4, NFκB, IκBβ, NOX4, and p-AMPK. AMPK, AMP-dependent kinase; FFA, free fatty acid; GIT27,4,5-dihydro-3-phenyl-5-isoxasole acetic acid; HG, high glucose; IκB, inhibitory kappa B; NFκB, nuclear factor kappa B; NG, normal glucose; NOX, nicotinamide adenine dinucleotide phosphate oxidase; SREBP1, sterol regulatory element binding transcription protein1; TLR, Toll-like receptor.
Discussion
In our study, we demonstrated that the TLR antagonist GIT27 improved obesity-related renal injury through the suppression of inflammatory changes in both the kidneys and the adipose tissue. GIT27, a novel immunomodulatory compound, modulates macrophage function and inhibits NFκB and proinflammatory cytokines through the TLR4 and TLR2/3 signaling pathways [19], [20]. Our study showed that GIT27 treatment in animals with HFD-induced obesity improved their metabolic disorders such as glucose intolerance and lipid abnormalities. Moreover, GIT27 treatment also decreased the urinary excretion of albumin and protein in obesity-related kidney disease, urinary oxidative stress markers, and inflammatory cytokine levels, and inhibited the expression of oxidative stress and proinflammatory cytokines in the kidneys and adipose tissue. In addition, GIT27 treatment improved extracellular matrix expansion and tubulointerstitial fibrosis in obesity-related kidney disease.
Recent works have proved that adipose tissue is a highly metabolic organ with pluripotent functions far beyond the mere storage of energy. Adipose tissue is now considered an endocrine organ that secretes a large number of bioactive cytokines, which have essential roles in energy homeostasis, glucose and lipid metabolism, insulin resistance, inflammation, and atherosclerosis [28], [29]. Obesity and its related metabolic diseases are believed to involve chronic low-grade inflammation characterized by the activation of proinflammatory cytokines and infiltration of macrophages into adipose tissue, leading to increased oxidative stress and insulin resistance. Molecular mechanisms underlying macrophage recruitment into adipose tissue are unclear. However, recent reports have revealed that stimuli such as LPS and FFAs activate the intracellular transcriptional NFκB pathway via the TNF receptor 1, IL-6 receptor, and TLR. Tremendous progress has been made to discover the role of the TLR family of proteins as a key component of the innate immunity system and in various inflammatory responses [30]. TLR4 is the most attractive candidate for investigating the connection between innate immunity and metabolic disorders [31], [32]. The NFκB signaling pathway in adipose tissue macrophages is considered to be a link between TLR4, obesity, and insulin resistance.
In our study, we confirmed that the number of F4/80-expressing cells increased in the kidney tissue of obese mice and decreased with GIT27 treatment. This result is interesting, despite a previous study investigating the role of macrophages in various kidney disease models [33]. Low-grade inflammation due to the infiltration of macrophages into the kidneys and adipose tissue has been shown to be induced by obesity and modulated by the TLR antagonist. GIT27 decreased urinary albumin excretion, oxidative stress, and activation of proinflammatory molecules in both the kidneys and adipose tissue of HFD mice. However, although these immunomodulatory effects of TLR antagonist on macrophages have already been known, its effect on the kidneys has rarely been reported. We hypothesized that GIT27 can show its direct effects on both kidney cells and adipocytes through inhibition of the TLR signaling pathway. As expected, in vivo data showed that GIT27 improved obesity-induced inflammatory changes in both the kidneys and adipose tissue. These results suggest that the TLR pathway may play a central role in the crosstalk between the kidneys and adipose tissue in obesity. However, we could not observe a notable change in TLR4 expression within the kidneys by GIT27 treatment. The expression of the TLR signaling pathway tended to increase in the kidneys of HFD mice and decrease in those treated with GIT27, but this difference was not significant. This may be explained by the fact that GIT27 is not a selective antagonist for TLR4 and has different cell- or organ-specific effects depending on specific diseases. Another explanation could be that it may exert a beneficial effect on the kidneys through an indirect mechanism of improvement of metabolic parameters such as glucose tolerance and lipid profiles. To investigate the direct effect of glucose and lipid stimulation on the kidney cells and adipocytes, we cultured podocytes and adipocytes and then treated them with HG and FFA. We confirmed TLR4 expression in cultured podocytes previously, and TLR4 mRNA expression was increased markedly under HG conditions in those cells [18]. Interestingly, in this study, HG and FFA stimulated a TLR signaling pathway in cultured podocytes. In particular, FFA increased the synthesis of NFκB and IκBβ, which were inhibited significantly by GIT27. However, these changes were not observed in mesangial cells (data not shown). These results suggest that the TLR antagonist can have its direct effect on the kidneys through podocyte injury. By contrast, the TLR antagonist did not affect adipocytes. The protein expression of SREBP1, TLR4, NFκB, IκBβ, NOX4, and p-AMPK in adipocytes was not changed significantly by FFA and GIT27. These results suggest that macrophage recruitment into the adipose tissue may have a more crucial role in obesity-induced immunomodulatory inflammatory mechanism than changes of adipocytes.
Our study presents the limitation that we could not identify a significant change of the TLR pathway in the kidneys of HFD mice. However, this result is interesting because an inhibition of TLR expression by the TLR antagonist was more remarkable in the kidneys of a diabetic animal model than in those of an obese animal model. We demonstrated previously that urinary nephrin excretion was more enhanced in diabetic mice than in nondiabetic mice, and GIT27 decreased nephrin excretion significantly in a podocyte injury model of diabetic nephropathy [18]. Lin et al [34] recently reported that the TLR4 antagonist CRX-526 attenuated renal injury through the TGFβ and NFκB pathways in advanced diabetic nephropathy. Both the studies proved that the TLR antagonist inhibited the expression of TLR pathway significantly in the kidneys of diabetic nephropathy animal. We also observed a dramatic change in the expression of NFκB and IκBβ in podocytes by FFA and GIT27. All these results suggest that the TLR antagonist improves obesity-induced kidney injury. In particular, their mechanism may at least partially be associated with the TLR signaling pathway in podocyte injury. This TLR signaling pathway may be related to the nephrin expression in podocyte injury [18]. Further studies may develop a TLR antagonist that is selective for TLR4 and specific to different organ and diseases, in order to eventually uncover the exact mechanisms involved.
In conclusion, the TLR antagonist GIT27 improved metabolic disorder and exerted renoprotective effects on obesity-related kidney disease through its anti-inflammatory properties. These effects were associated with the TLR pathway of podocyte injury. Further study to elucidate the renoprotective mechanism of a TLR antagonist is under investigation. In the near future, GIT27 may be used in the treatment of multiple renal diseases associated with innate immunity.
Conflicts of interest
The authors declare no conflicts of interest.
References
Acknowledgments
This study was supported by a grant from Korea University.
