Long-term effectiveness of cinacalcet in non-dialysis patients with chronic kidney disease and secondary hyperparathyroidism
Article information
Abstract
Background
Secondary hyperparathyroidism (SHPT) is a common complication of chronic kidney disease (CKD). Cinacalcet use is controversial in non-dialysis patients.
Methods
This retrospective observational study recruited patients receiving cinacalcet (off-label use) in 2010 and 2011. Patients were followed for three years from the beginning of treatment using an intention-to-treat approach.
Results
Forty-one patients were studied: 14 CKD stage 3 (34.1%), 21 CKD stage 4 (51.2%), and 6 CKD stage 5 (14.6%). Median baseline parathyroid hormone (PTH) was 396 (101–1,300) pg/mL. Upon cinacalcet treatment (22 ± 12 months), PTH levels decreased by ≥ 30% in 73.2% of patients (P < 0.001; 95% confidence interval [CI], 59–87%), with a mean time for response of 18.7 months (95% CI, 15.4–22.1). Sixteen patients were followed for 36 months and treated for 32 ± 9 months. Mean reduction in their PTH levels was 50.1% (P < 0.001; 95% CI, 33.8–66.4%) at 36 months, with 62.5% of patients (P < 0.001; 95% CI, 35.9–89.1%) presenting reductions of ≥ 30%. Serum calcium levels decreased from 9.95 ± 0.62 mg/dL to 9.21 ± 0.83 and 9.12 ± 0.78 mg/dL at 12 and 36 months, respectively (P < 0.001). Serum phosphorus levels increased from 3.59 ± 0.43 to 3.82 ± 0.84 at 12 months (P = 0.180), remaining so at 36 months (P = 0.324). At 12 and 36 months, 2 (12.5%) patients experienced hypocalcemia. Meanwhile, 1 (6.3%) and 4 (25.0%) patients reported hyperphosphatemia at 12 and 36 months, respectively.
Conclusion
Cinacalcet remained effective for at least 36 months in non-dialysis patients with SHPT. Electrolytic disturbances were managed with concurrent use of vitamin D and its analogs or phosphate binders.
Introduction
Secondary hyperparathyroidism (SHPT) is a frequent complication in patients with chronic kidney disease (CKD) that appears in the early stages of the disease and is exacerbated by worsening kidney function [1]. Sustained overproduction of parathyroid hormone (PTH) induces a systemic disorder characterized by high bone turnover and vascular calcification, which increase the risk of bone fracture and cardiovascular mortality [2,3].
Treatment of SHPT aims to decrease PTH levels while maintaining calcium and phosphorus serum levels within normal ranges [4]. Cinacalcet (Amgen, Thousand Oaks, CA, USA), as well as vitamin D and its analogs, phosphate binders, parathyroidectomy, and a restricted dietary intake of phosphorus, can be used to slow the progression of SHPT [1–4]. Cinacalcet is a type II calcimimetic that decreases PTH synthesis and release by increasing the sensitivity of calcium-sensing receptors in the parathyroid chief cells to extracellular calcium [4].
Cinacalcet is currently indicated for the treatment of SHPT in patients with end-stage renal disease undergoing dialysis [5]. It has been shown to remain effective for at least 3.5 years of treatment [6,7].
The treatment of SHPT with cinacalcet has also been evaluated in non-dialysis patients [8–16]. In a phase III clinical trial, cinacalcet decreased PTH levels by 43%. However, it also caused hypocalcemia in 62% of patients, although they were mostly asymptomatic. More worryingly, it increased phosphorus serum levels by 21.4% [8]. Similar or greater reductions in PTH levels were also observed in other clinical practice studies [10–16].
SHPT is a chronic and progressive condition that worsens over time, even in non-dialysis patients. Consequently, prolonged treatment is required. However, the effectiveness of cinacalcet has only been evaluated in the short term. The long-term effectiveness and safety of cinacalcet in non-dialysis patients have not yet been studied.
Therefore, our aim in this study was to evaluate the long-term effectiveness and safety of cinacalcet in patients with CKD and SHPT who had not received renal replacement therapy (RRT) or renal transplantation (RT).
Methods
Materials and methods
An analytical, retrospective, observational, non-placebo-controlled and multi-center lead-in study was conducted and previously reported elsewhere [10,11]. The lead-in study followed patients for up to 12 months. The same patients were then followed for up to 36 months in this extension study.
The inclusion criteria were adults diagnosed with CKD and SHPT who had not received RRT or RT and had taken cinacalcet, under off-label use, from the Outpatient Pharmacy Service (OPS) during 2010 and 2011 and then continued cinacalcet treatment for up to 36 months before beginning RRT (hemodialysis or peritoneal dialysis) or RT. SHPT was diagnosed if PTH serum levels were sustained for 6 months above 200 pg/mL with Ca serum levels above 9 mg/dL. The exclusion criterion was participation in an active cinacalcet clinical trial. The cinacalcet prescription criteria were patients with elevated PTH levels or those presenting a sharp increase in PTH levels. Individualized dosage adjustments were made as needed based on each patient’s biochemical values. Patients first received standard treatment if their serum calcium levels allowed it [10,11]. The Clinical Research Ethics Committees of the Vall d’Hebron University Hospital and Bellvitge University Hospital approved this study (approval no. CEIC VH-3772). Neither written nor verbal consent for study participation was systematically obtained. The study was non-interventional and retrospective, using routine data collected from clinical records. Moreover, most of the patients were outpatients, which made them difficult to find and contact. Verbal consent was obtained when feasible from patients who made routine visits to the hospital outpatient setting during the study period. The Ethics Committee approved this procedure, which is routine for observational retrospective studies. To protect patient privacy, anonymized data are available on request from the corresponding author.
The patients were followed for up to 36 months from the beginning of cinacalcet treatment using medical data collected every 3 months. As in the lead-in study, the compiled information contained biodemographic data; therapeutic details about the use of cinacalcet, vitamin D and its analogs, phosphate binders, and calcium supplements; and biochemical values for PTH, serum calcium and phosphorus, albumin, 25-OH-vitamin D, serum and urine creatinine, proteinuria, alkaline phosphatase, and gamma-glutamyl-transferase [10,11]. Serum PTH levels were measured by Liaison XL (DiaSorin, Stillwater, USA) and Immulite 2000 XPi (Siemens Healthcare, Erlangen, Germany) at Vall d’Hebron University Hospital and Bellvitge University Hospital. The cinacalcet dose was reported as the average weekly dosage.
The long-term effectiveness of cinacalcet was measured by analyzing changes during the follow-up period in the number of patients with reductions in their PTH levels of 30% or more from their baseline values. Secondary effectiveness outcomes included changes in mean PTH levels from baseline to 36 months. Long-term safety was assessed by measuring changes in the mean serum levels of calcium and phosphorus and incidences of hypocalcemia (two consecutive calcium readings of < 8.4 mg/dL) and hyperphosphatemia (phosphorus > 4.5 mg/dL) [3] from baseline to 36 months. The reasons for stopping cinacalcet treatment were also recorded.
Either the Cockcroft and Gault [17] or the Modification of Diet in Renal Disease [18] study equation was used to stratify patients by their estimated glomerular filtration rate (eGFR): CKD stage 3, 30–59 mL/min/1.73 m2; CKD stage 4, 15–29 mL/min/1.73 m2; and CKD stage 5, < 15 mL/min/1.73 m2.
Statistical analysis
The principal analysis was conducted using the intention-to-treat approach. To evaluate the effectiveness of the primary and secondary variables, the student-Fisher t test was used for continuous variables with a normal distribution, and a variance test such as two-way ANOVA was used for repeated measures. The Friedman or Kruskal–Wallis test was used to compare variables that did not follow a normal distribution. Fisher’s exact test was applied for categorical variables. Because 36 months of follow-up were not achieved for every study participant, a survival analysis was also performed. Confidence intervals (CIs) were calculated using a Student t-paired test with α = 0.05. The number of patients was not predefined because all the patients with available records from our OPS in 2010 and 2011 were considered for the study.
In the safety analysis, all patients were included because all of them had received at least one dose of cinacalcet. Adverse events were tabulated by their incidence and severity. Adverse reactions were presumed to be related to cinacalcet treatment. Calcium and phosphorus serum levels were evaluated in a way similar to that used for the efficacy variables. Statistical analyses were conducted using SPSS ver. 15.0 software (SPSS Inc., Chicago, USA).
Results
A total of 41 patients were evaluated in the lead-in study, and their baseline characteristics are summarized in Table 1. The patients were followed up for a mean period of 26 ± 9 months and were treated with cinacalcet for a mean period of 22 ± 12 months. Sixteen of them were followed for 36 months, and those patients received cinacalcet for a mean period of 32 ± 9 months. Their demographic and analytic characteristics are shown in Table 1 and 2. Twenty-five patients had a shorter follow-up period because the study had closed (48.0%) or because they initiated RRT (44.0%), were lost to follow-up (4.0%), or died (4.0%). However, 68% of patients were still on cinacalcet treatment on their last recorded visit.
At the end of the study, the mean cinacalcet dose was 204.38 ± 154.62 mg/week (29 mg/day). The most common daily doses of cinacalcet were < 30 mg/day (42.9%) and 30 to 60 mg/day (28.6%), followed by 30 mg/day (14.3%), 60 mg/day (7.1%), and > 60 mg/day (7.1%). For patients receiving < 30 mg/day, the most common dose was 30 mg of cinacalcet three times a week (14.5%), followed by 30 mg five times a week (7.1%), 30 mg six times a week (7.1%), 30 mg weekly (7.1%), and other combinations (7.1%). At the end of the study, common daily doses of cinacalcet were ≤ 30 mg/day in the 60.0% and 55.5% of patients who started with CKD 3 and CKD 4, respectively. Patient who started with CKD 5 and ended the study with cinacalcet treatment used 60 mg/day. At baseline, 43.8% and 18.8% of patients used vitamin D and its analogs and phosphate binders, respectively, increasing to 62.5% and 18.8% of patients at 12 months and 87.5% and 25.0% of patients at the end of the study. None of the patients used calcium supplements. At the end of the study, the vitamin D and its analogs used were calcitriol (37.5%), calcifediol (25.0%), paricalcitol (25.0%), and alfacalcidol (6%), and some of the phosphate binders used contained calcium (25.0%).
The survival analysis showed that 73.2% (P < 0.001; 95% CI, 59–87%) of patients achieved at least a 30% reduction in their PTH levels during the follow-up period. The mean time for response to treatment (PTH reduction > 30%) was 18.7 months (95% CI, 15.4–22.1 months) (Fig. 1).
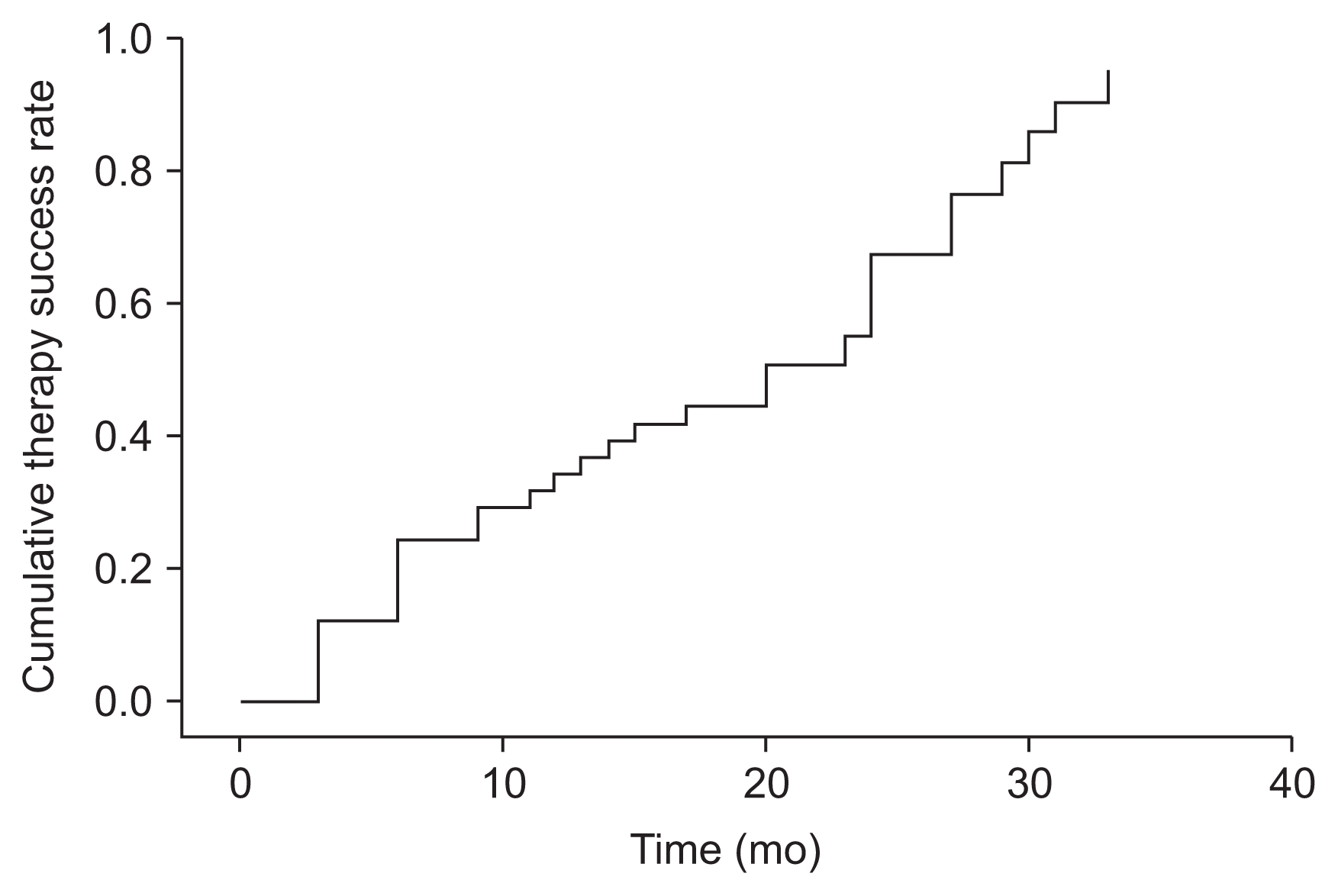
Survival therapy success, measured as achieving a reduction of ≥ 30% in the parathyroid hormone level (n = 41).
For patients followed for 36 months, the mean reduction in PTH levels was 50.1% (P < 0.01; 95% CI, 33.8–66.4%), with 62.5% of patients (P < 0.001; 95% CI, 35.9–89.1%) presenting at least a 30% reduction in their PTH levels (Table 3). Cinacalcet significantly reduced PTH levels in the first year of treatment and then maintained that reduction for up to 36 months. A marked decrease was observed after just three months of cinacalcet treatment. Changes in PTH levels over the 36 months of treatment are shown in Fig. 2. No significant differences were observed in the effectiveness of cinacalcet among the groups with different stages of CKD (Table 4).
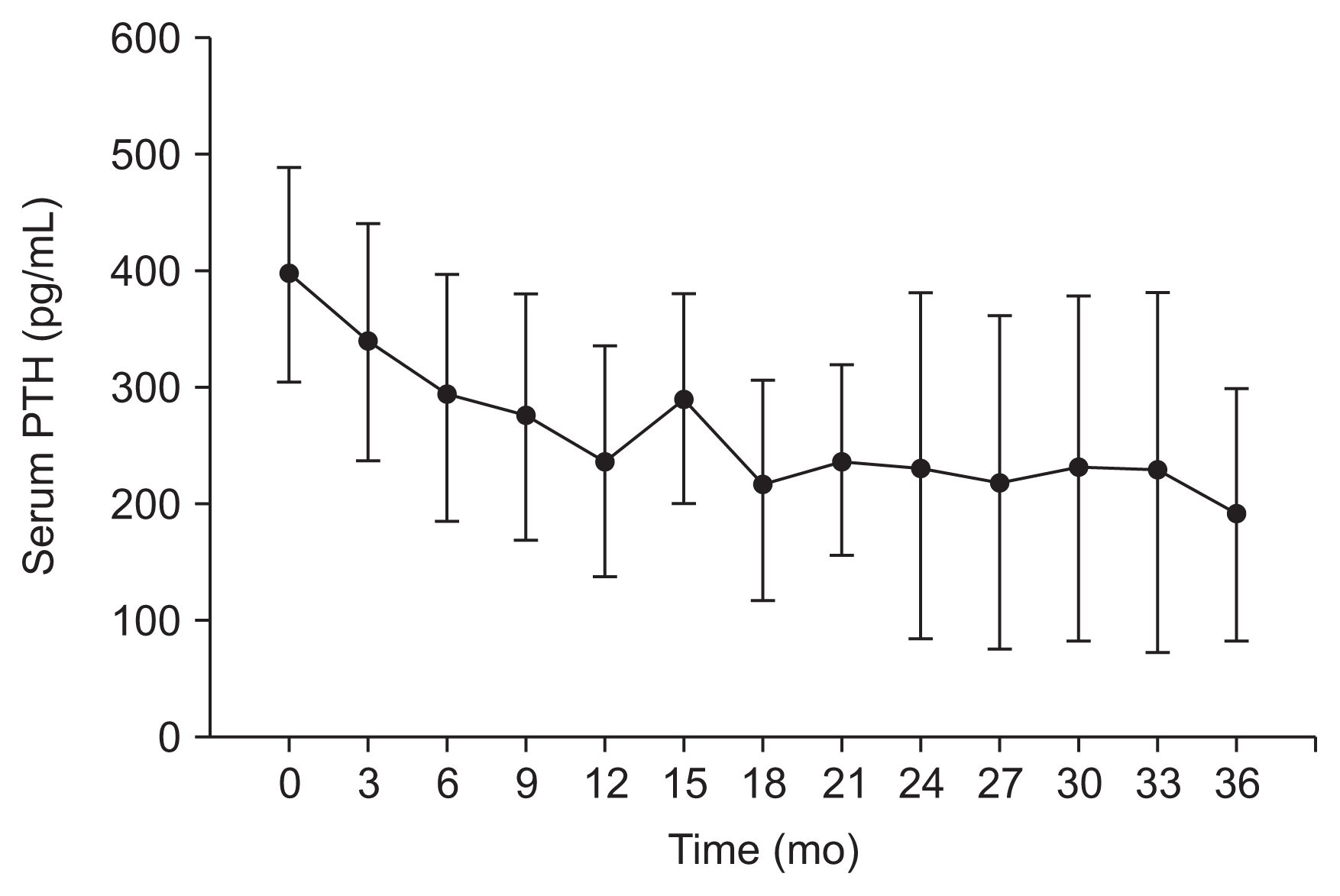
Changes in parathyroid hormone (PTH) levels over 36 months of cinacalcet treatment (n = 16; mean, 95% confidence interval [CI]).

Effectiveness outcomes during 36 months of cinacalcet therapy: results according CKD stages (n = 16)
Cinacalcet reduced calcium levels and increased phosphorus levels, as can be seen in Fig. 3 and 4, respectively. At baseline, the mean calcium level was 9.95 ± 0.62 mg/dL, which was reduced by 7.4% at 12 months (P < 0.001) and 8.3% at 36 months (P < 0.001). At baseline, the mean phosphorus level was 3.59 ± 0.43 mg/dL, which increased, without statistical significance, by 6.3 and 5.7% at both 12 months (P = 0.180) and 36 months (P = 0.324).
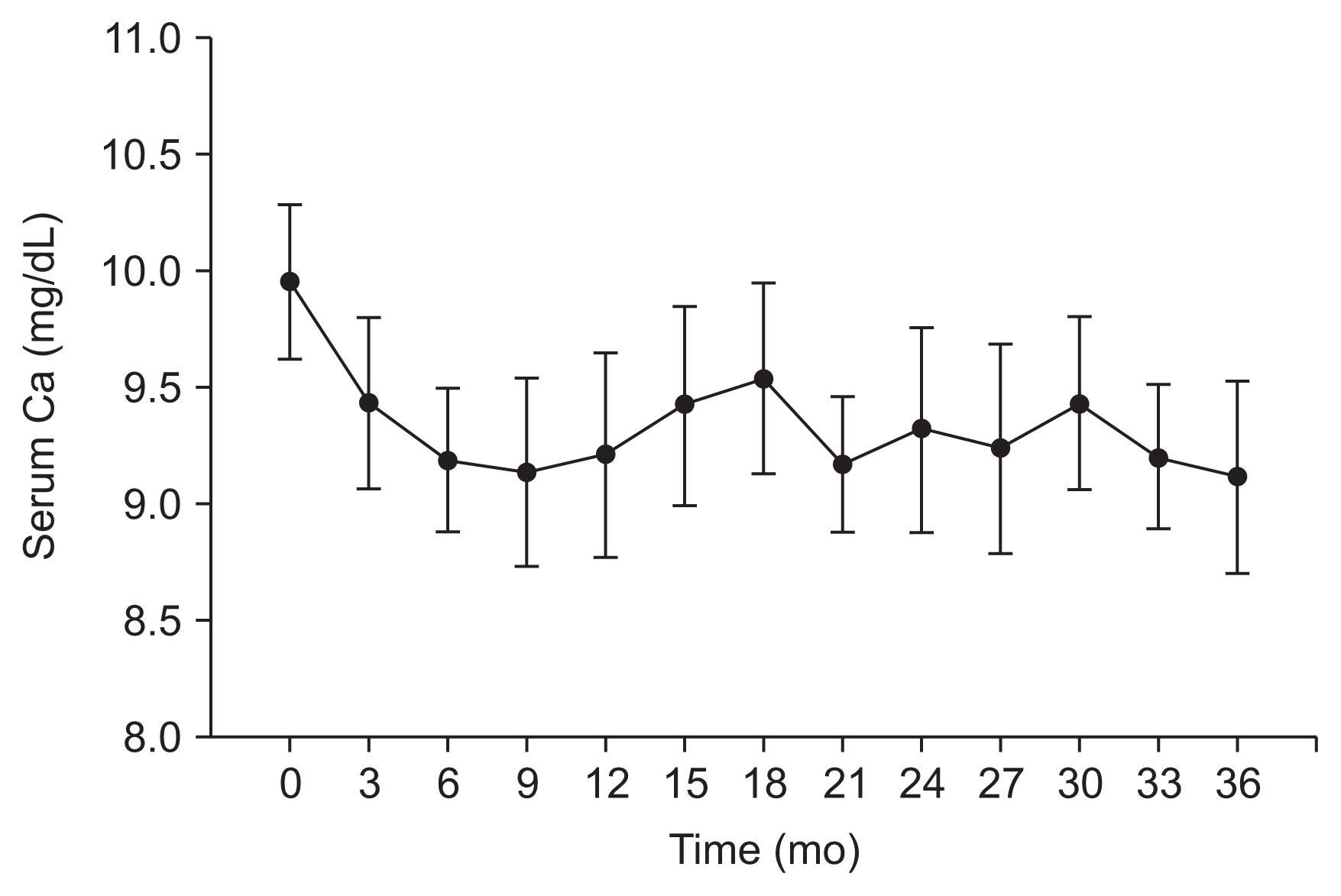
Changes in calcium (Ca) levels over 36 months of cinacalcet treatment (n = 16; mean, 95% confidence interval [CI]).
Cinacalcet treatment induced hypocalcemia and hyperphosphatemia. No patients presented hypocalcemia at baseline, whereas 2 (12.5%) patients did at 12 months. Hypocalcemia persisted for up to 36 months of cinacalcet treatment. Corrective measures consisted of decreasing or stopping cinacalcet treatment and/or increasing the uptake of vitamin D and its analogs. Similarly, no patients presented hyperphosphatemia at baseline, but 1 (6.3%) and 4 (25.0%) patients did at 12 months and 36 months, respectively.
Cinacalcet treatment was discontinued by 25% of patients before 36 months, although 12.5% of them went on to restart treatment. One patient stopped twice. The reasons for withdrawing treatment were: hypocalcemia (6.3%), non-compliance (6.3%), interactions (6.3%), other adverse reactions (6.3%), and unknown (6.3%). Compared to the overall population of the lead-in study [10,11], treatment withdrawal occurred mainly in the first 12 months of treatment (Table 5). One patient treated with cinacalcet died after admission to a hospital with a gastrointestinal hemorrhage whose cause could not be assessed. In addition, the patient showed acidosis and during hospitalization presented ventricular fibrillation with QT prolongation. Eco-Doppler showed moderate aortic stenosis, severe mitral insufficiency, and moderate ventricular dysfunction. At the time of hospitalization, the patient was receiving furosemide, allopurinol, amiodarone, calcitriol, cinacalcet, omeprazole, calcium/vitamin D supplement, atorvastatin, and pegylated epoetin beta, and their featured analytical values were 3,89 mg/dL potassium, 1.9 mg/dL magnesium, and 7.7 mg calcium.
Patients in the lead-in and extension studies experienced adverse events that did not stop them from continuing cinacalcet treatment: 4 patients reported gastrointestinal discomfort; 1 presented muscle spasms, 1 experienced paresthesia, and 1 reported alopecia. Most of those adverse events occurred in the first 12 months of cinacalcet treatment [10,11].
Discussion
To the best of our knowledge, this is the first study to evaluate the long-term effectiveness and safety of cinacalcet in a reasonable sample size of non-dialysis patients. We not only analyzed changes in biochemical values for 36 months, but we also carried out a survival analysis.
The survival curve showed that the mean time for achieving a reduction of ≥ 30% in the PTH level in non-dialysis patients with SHPT was 18.7 months (95% CI, 15.4–22.1 months). In the lead-in study [10,11], we observed that 72.7% of patients with baseline PTH levels > 300 pg/mL presented reduced PTH levels as early as in the first three months of cinacalcet treatment. By contrast, only 16.7% of patients with baseline PTH levels < 300 pg/mL showed the same reductions in their PTH levels. However, 58.3% of patients with a baseline PTH level < 300 pg/mL displayed a reduction of ≥ 30% in their PTH levels after 12 months of cinacalcet treatment [10,11], which explains the results found in this extension study.
NICE recommends the continuation of cinacalcet treatment in hemodialysis patients if the patients achieve a reduction of ≥ 30% in their PTH levels after four months of treatment [19]. Our study evaluated non-dialysis patients with a mean PTH concentration of 396 (101–1,300) pg/mL in the lead-in study [10,11] and 354 (197–837) pg/mL in this extension study. These PTH levels are greater than those found in other epidemiological studies in non-dialysis patients, which reported values of 67 to 90 pg/mL, 90 to 160 pg/mL, and 220 to 250 pg/mL in patients with CKD3, CKD4, and CKD5, respectively [20–22]. In addition, our mean PTH level was far above the NKF Kidney Disease Outcomes Quality Initiative (NKF-K/DOQI) recommendations [3] and even greater than the baseline PTH levels for CKD3 and CKD4 observed in a previous phase III clinical trial [8]. Moreover, PTH levels negatively correlate with eGFR [2,3], indicating that our patients would present increasing PTH levels over time. PTH levels also correlate with high bone turnover [23], increasing the fluid loss of calcium and phosphorus [4,12,24], which is, in turn, associated with soft tissue calcification [25] and cardiovascular morbidity and mortality [2,3]. Therefore, non-dialysis patients with high PTH levels could be considered high-risk patients. It would be interesting to debate how long clinicians should be prepared to wait to achieve a PTH reduction of 30% in such high-risk patients.
This study found that cinacalcet is not only effective in the short term [8–16], but also in the long-term in patients not yet on dialysis. In this study, 50.0% of patients achieved at least a 30% reduction in their PTH level, which increased slightly to 62.5% of patients with 36 months of follow-up data. The overall mean reduction in PTH levels was 38.9%, becoming 50.1% at 36 months. Thus, cinacalcet was effective for at least 36 months. These results are consistent with those from long-term studies of cinacalcet in hemodialysis patients [6,7]. Moreover, we observed that cinacalcet significantly reduced PTH levels after three months and was equally effective for all stages of CKD, which agrees with the results of the lead-in study [10,11].
Cinacalcet maintained its effectiveness without requiring a large increase in its dose. In this study, the cinacalcet dose increased by 29.8% from baseline to 36 months, but it still remained close to the recommended starting dose for cinacalcet in hemodialysis patients [5]. These findings were similar to those obtained by Sprague et al [6] in dialysis patients, in whom cinacalcet remained effective for at least 3.5 years without considerable increases in dosage. Furthermore, cinacalcet was previously reported to maintain PTH levels for 24 months in 8 non-dialysis patients [13].
The present study might have recruited a higher number of cinacalcet responders. Compared to the lead-in study, this study had a higher proportion of male patients, patients with diabetes mellitus, younger patients, and patients with lower baseline PTH levels. Some authors have reported that diabetes mellitus could decrease PTH secretion [26], and Kim et al [27] found that non-responders to cinacalcet tended to be younger and male. However, factors affecting cinacalcet response remain unclear. The main reasons for losing patients in this extension study were study closure and patients starting RRT. However, many patients continued cinacalcet after starting RRT. Thus, patient losses did not appear to affect the results of this study.
In this study, mean serum calcium levels decreased at the onset of cinacalcet treatment but remained stable from 12 to 36 months. The number of hypocalcemia cases also remained stable from 12 to 36 months. Cinacalcet-related hypocalcemia has been widely reported [5]. Hypocalcemia seems to be linked to the reduction in PTH levels, which decreases the release of calcium from bones [4] and increases calcium excretion from the kidneys [12,24]. Hypocalcemia is the most concerning adverse effect of cinacalcet treatment in non-dialysis patients. However, it can be treated by elevating the intake of vitamin D and its analogs, which stimulates the intestinal absorption of calcium [1] and could prevent hypocalcemia. At 36 months, 87.5% of patients received vitamin D and its analogs, although the sharpest increase in the incidence of hypocalcemia occurred in the first three months of cinacalcet treatment.
Another worrying issue is the short-term increase in phosphorus levels reported in non-dialysis patients on cinacalcet. CKD3 and CKD4 patients have residual renal function that could increase the tubular reabsorption of phosphorus due to the decreased PTH levels [12,24]. Elevated phosphorus levels are the primary cause of vascular calcification [25]. Palmer [28] demonstrated that the all-cause mortality risk increased by 18% for each 1-mg/dL increase in the phosphorus level (relative risk [RR], 1.29; 95% CI, 1.12–1.48) in non-dialysis patients; the correlation was more consistent when phosphorus levels were > 5.5 mg/dL [28], although other studies have reported similar findings for phosphorus levels > 7 mg/dL [29].
In this study, we observed an increased number of hyperphosphatemia cases between 12 and 36 months. Unlike the lead-in study [10,11], this study did not find any baseline episodes of hyperphosphatemia. The loss of patients due to their initiation of RRT did not influence these results because hyperphosphatemia was not a main or routine reason to start RRT [30]. Surprisingly, mean phosphorus levels decreased and remained stable between 15 and 36 months, reversing the trend observed during the first 15 months of cinacalcet treatment.
Our observations suggested two types of patients. Some patients displayed increased phosphorus levels upon cinacalcet treatment in both the short- and long-term. In addition, these increases were favored by a high intake of vitamin D and its analogs, which stimulate the intestinal absorption of phosphorus [1]. By contrast, phosphorus levels in some patients were unaffected or decreased in response to cinacalcet, possibly because of better control of PTH levels. Rodriguez et al [31] showed a strong association between PTH and phosphorus concentrations, and Frazão et al [32] demonstrated better control of phosphorus levels in dialysis patients when PTH levels were effectively decreased by cinacalcet or conventional treatment with vitamin D and its analogs and/or phosphate binders [32]. Consequently, better control of PTH levels through cinacalcet treatment might have maintained or decreased the phosphorus levels in some non-dialysis patients in our study. Deteriorating renal function could also explain the decrease in phosphorus levels because phosphorus reabsorption requires residual renal function [12,24]. However, in this study, eGFR did not significantly decrease throughout the 36 month study period.
We noted that cinacalcet was well tolerated for at least 36 months, with patients experiencing the most adverse events during the first 12 months of treatment. However, more studies are needed to evaluate the incidence of adverse events with cinacalcet therapy over time. Hypocalcemia was the main reason for withdrawing cinacalcet treatment, whereas gastrointestinal discomfort did not lead to the discontinuation of cinacalcet treatment in most cases.
This study has some limitations. It was observational and retrospective. Therefore, relevant data might have been lost, and bias could have been introduced. This study analyzed surrogate parameters rather than hard clinical outcomes. However, cinacalcet treatment achieved a sustained PTH reduction over time and seemed to maintain phosphorous values during 36 months in some patients. This study is the first to follow up non-dialysis patients on cinacalcet for up to 36 months; however, few patients were followed that long. Finally, the eGFR was calculated using two different equations, and PTH levels were measured using two different analyzers. Nevertheless, the results were not affected by those differences because the P significance value was far from 0.05 in most cases. Finally, urinary indices of calcium and phosphorus could not be assessed due to the lack of available data.
In conclusion, cinacalcet maintained reduced PTH levels for at least 36 months in non-dialysis patients. This effectiveness did not require a large increase in the cinacalcet dose. However, calcium and phosphorus levels should be monitored over time to prevent hypocalcemia and hyperphosphatemia. Hypocalcemia can be managed with the administration of vitamin D and its analogs. Cinacalcet treatment was associated with an increased number of hyperphosphatemia cases; however, phosphorus levels remained unaffected or were reduced in some patients. Prospective studies are needed to corroborate our observations and study the effects of cinacalcet on cardiovascular morbidity and mortality in non-dialysis patients.
Acknowledgments
This work was funded by a grant from Col·legi Oficial de Farmacèutics de Barcelona. Col·legi Oficial de Farmacèutics de Barcelona was not involved in the design of the protocol, participant recruitment, data analysis and interpretation, or the writing of the manuscript. Some of the data included in this work were presented at the 61st Congreso de la Sociedad Española de Farmacia Hospitalaria in October 2016 in Gijón, Spain, in an abstract and poster format.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Author’s contributions
Ariadna Pérez-Ricart, José-Bruno Montoro-Ronsano: Conception or design, analysis and interpretation of data, or both; Ariadna Pérez-Ricart, Maria Galicia-Basart, Josep-Maria Cruzado-Garrit, Alfons Segarra-Medrano, José-Bruno Montoro-Ronsano: Drafting or revising the article; Ariadna Pérez-Ricart, Dolors Comas-Sugrañes, Alfons Segarra-Medrano, José-Bruno Montoro-Ronsano: Providing intellectual content of critical importance to the work described; Ariadna Pérez-Ricart, Maria Galicia-Basart, Dolors Comas-Sugrañes, Alfons Segarra-Medrano, José-Bruno Montoro-Ronsano: Final approval of the version to be published.




