Stabilization of serum alkaline phosphatase in hemodialysis patients by implementation of local chronic kidney disease-mineral bone disorder management strategy: A quality improvement study
Article information
Abstract
Background
The aim of this study is to narrow the gap between global guidelines and local practices, we recently established domestic recommendations by adapting the international guidelines for management of chronic kidney disease-mineral bone disorder (CKD-MBD) in patients on maintenance hemodialysis (MHD). This study was undertaken to determine whether application of this guideline adaptation was associated with improved serum mineral profiles in patients with CKD-MBD.
Methods
A total of 355 patients on MHD were enrolled from seven dialysis units. After adhering to our strategy for one year, serum phosphorus, calcium, intact parathyroid hormone (iPTH), and alkaline phosphatase (AP) levels were compared with the baseline. The endpoint was improvement in the proportion of patients with serum mineral levels at target recommendations.
Results
The median serum phosphorus level and proportion of patients with serum phosphorus within the target range were not changed. Although the median serum calcium level was significantly increased, the proportion of patients with serum calcium within the target range was not significantly affected. The proportion of patients with serum iPTH at the target level was not altered, although the median serum iPTH was significantly decreased. However, both median serum AP and the proportion of patients with serum AP at the target level (70.4% vs. 89.6%, P < 0.001) were improved.
Conclusion
In our patients with MHD, serum mineral profiles were altered and the serum AP level stabilized after implementing our recommendations. Long-term follow-up evaluations are necessary to determine whether uremic bone disease and cardiovascular calcifications are affected by these recommendations.
Introduction
Dysregulation of mineral and bone metabolism is common in patients on maintenance hemodialysis (MHD) and is typically characterized by abnormal serum calcium, phosphorus, parathyroid hormone (PTH), and alkaline phosphatase (AP) levels. International clinical practice guidelines, including the Kidney Disease: Improving Global Outcomes (KDIGO) and Kidney Disease Outcomes Quality Initiative (KDOQI), have suggested target ranges of serum minerals to prevent and/or ameliorate uremic bone disease and vascular calcification [1–3]. However, only about half of patients on MHD in different countries were reported to be within the guideline range for serum phosphorus and calcium [4].
In contrast, regional guidelines from Japan (Japanese Society for Dialysis Therapy, JSDT) [5], Australia (Caring for Australians with Renal Impairment, CARI) [6], United Kingdom (United Kingdom Renal Association, UKRA) [7], and Europe (European Renal Best Practice, ERBP) [8] presented variable target levels of serum phosphorus, calcium, and PTH for management of chronic kidney disease-mineral bone disorder (CKD-MBD). Furthermore, each country has its own reimbursement system that affects daily practices. This led us to prepare our own regional recommendations for CKD-MBD management in patients on MHD [9].
This study was undertaken to test whether our adapted domestic recommendations are feasible and valid in the management of our patients on MHD. The short-term effects on serum mineral levels were determined after applying a 1 year quality improvement (QI) strategy.
Methods
Patients
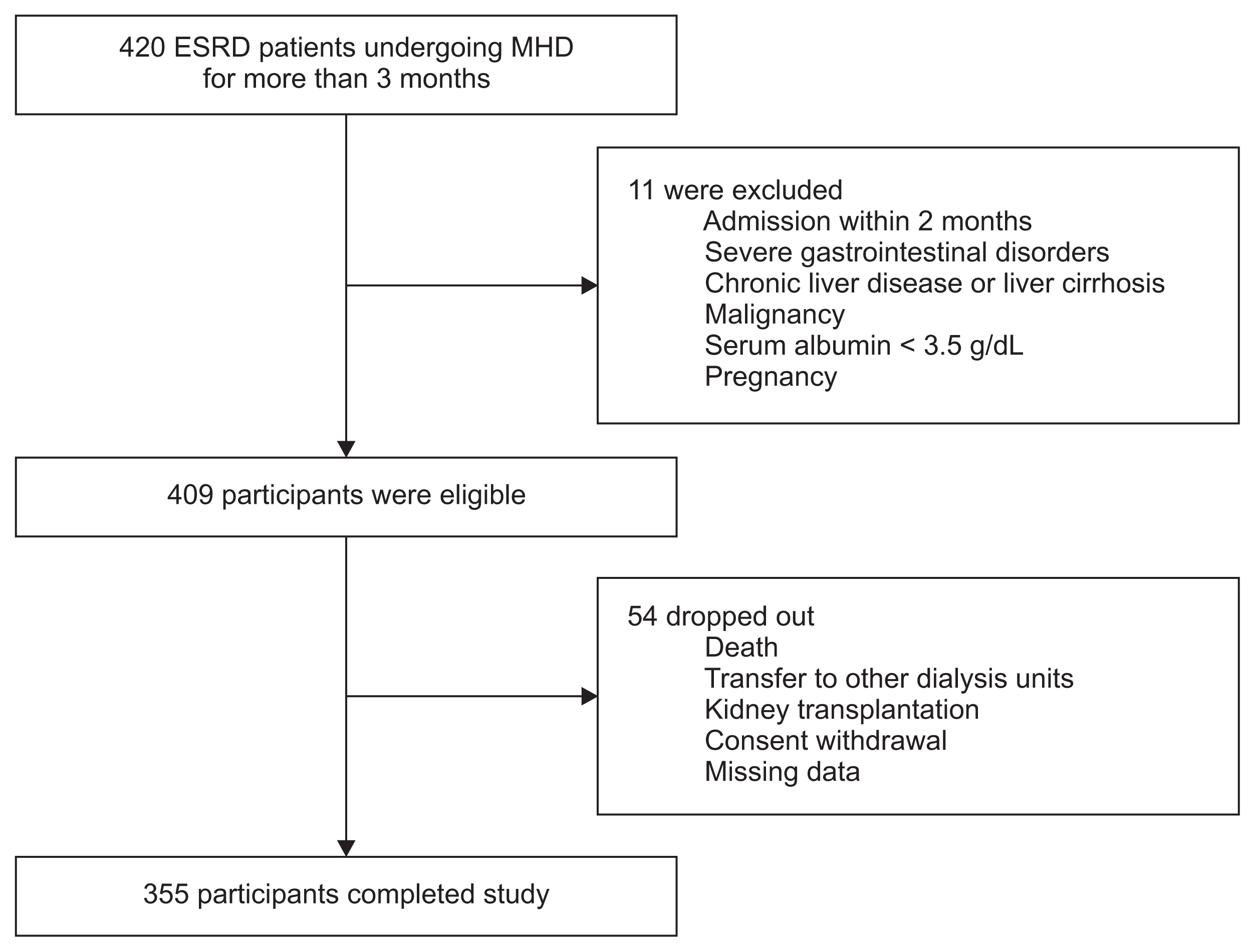
This study was conducted in seven medical centers in South Korea from March 2016 to February 2017. Adult (age ≥ 19 years) patients with end-stage renal disease (ESRD) who had been receiving MHD therapy for more than 3 months were screened between December 2015 and February 2016, and a total of 420 patients were identified. Eleven patients were excluded because of intercurrent illness requiring hospitalization during the prior 2 months, severe gastrointestinal disorders, chronic liver disease or liver cirrhosis, malignancy, serum albumin < 3.5 g/dL, and pregnancy. The remaining 409 patients were eligible, but 54 dropped out of this study due to death, transfer to other dialysis units, kidney transplantation, consent withdrawal, or no follow-up data. Therefore, 355 patients were included in the final analysis (Fig. 1). This study was approved by the review board of Hanyang University Hospital (IRB File No. 2015-10-045-004). Informed consent was obtained from each patient in accordance with the Helsinki Declaration.
Intervention and endpoints
During the study period, our previous recommendations for management of CKD-MBD were followed [9]. Fig. 2 summarizes our strategy to control hyperphosphatemia and secondary hyperparathyroidism. The choice of phosphate binders and PTH-lowering agents was determined based on serum calcium levels (Fig. 2A). Serum phosphorus, calcium, and AP were measured at least once per month, and the intact PTH level was measured once every 3 months. Our target ranges were as follows: serum phosphorus, 2.4 to 5.0 mg/dL; calcium, 8.4 to 9.6 mg/dL; and intact PTH, 100 to 300 pg/mL [10]. Prescriptions were altered monthly according to the flow chart shown in Fig. 2B.
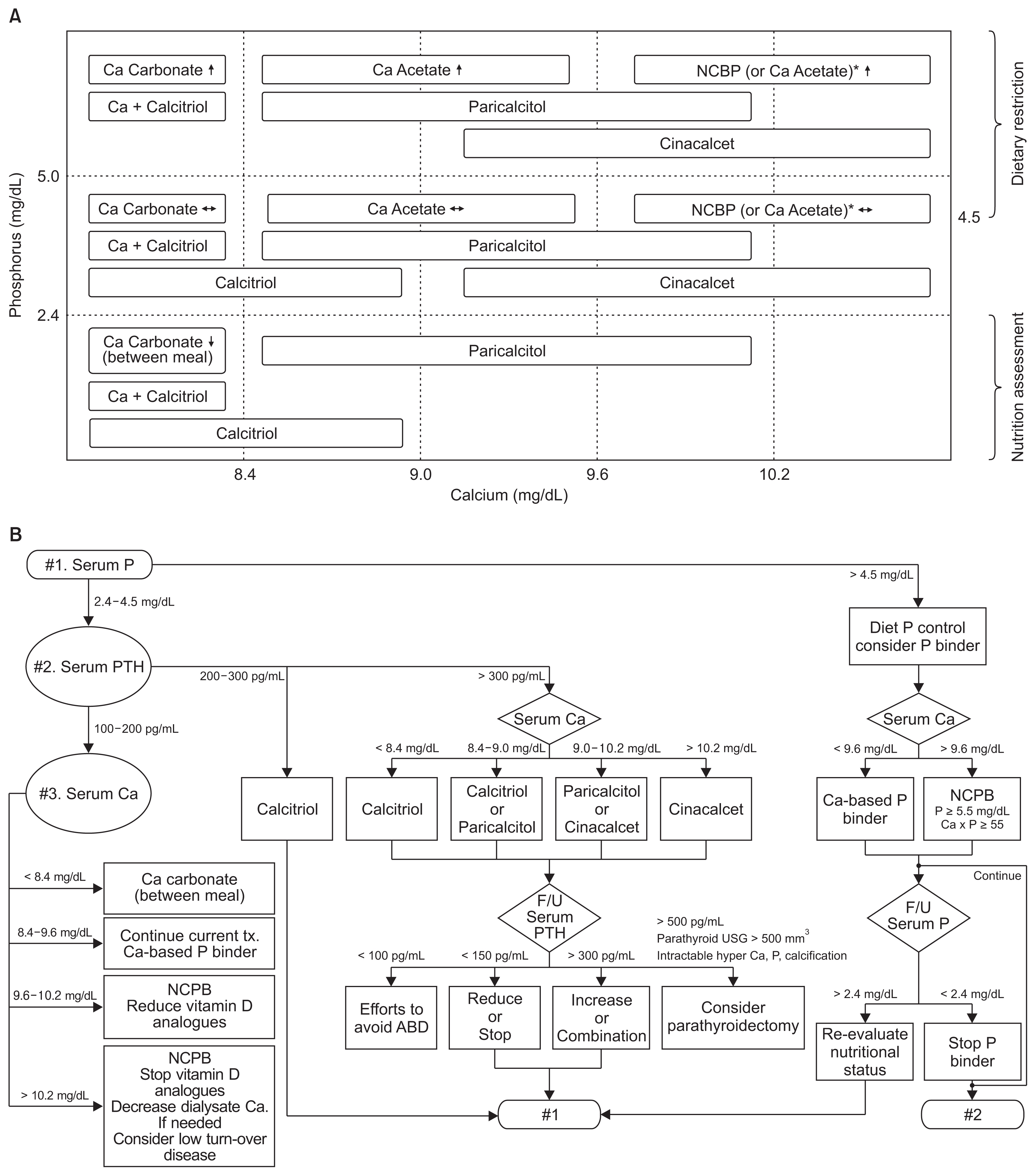
Quality improvement strategy for chronic kidney disease-mineral bone disorder (CKD-MBD) management based on Korean Recommendations
(A) Medication categories are shown according to levels of serum calcium and phosphorus. Adapted from the article of Hwang et al (Kidney Res Clin Pract 34:4–12, 2015) [9]. (B) Sequential approaches are presented for selection and adjustment of medications.
ABD, adynamic bone disease; Ca, calcium; F/U, follow-up; NCPB, non-calcium-based phosphate binder; P, phosphorus; PTH, parathyroid hormone; tx., treatment; USG, ultrasonography.
*Non–calcium-based phosphate binder (NCPB) is reimbursed for P ≥ 5.5 mg/dL and Ca-P ≥ 55 mg2/dL2 according to the current Korean National Health Insurance Standards.
After adhering to this strategy for 1 year, serum phosphorus, calcium, AP, and intact PTH levels were compared with the baseline. The primary endpoints of this study were improvement in the proportion of patients with serum mineral levels within the target range of our recommendations. The target level of serum AP was defined as ≤ 120 U/L in this study. Secondary endpoints were changes in serum mineral levels after introduction of our recommendations. Serum concentrations of phosphorus, calcium, and AP were determined by automated and standardized methods, and the intact PTH concentration was measured by second-generation PTH assays at each center. In addition to demographic features, data for use of calcium-based or calcium-free phosphate binders, vitamin D analogues, and cinacalcet were collected.
Statistics
Continuous data are presented as means ± standard deviation, and categorical variables are expressed as frequency counts and percentages. A McNemar test was used to assess the difference between the proportion of patients with serum mineral levels at the target of our recommendations before and after implementation of the QI strategy. A Wilcoxon signed-rank test was used to test the difference between basal and follow-up levels of serum minerals. Other categorical variables were also compared using the McNemar test. Statistical significance was defined as P < 0.05. All statistical analyses were performed using R programming language ver. 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Demographic features of patients
Table 1 shows demographic features of the patient population in our study, which consisted of 174 men (49.0%) and 181 women (51.0%) with a mean age of 55.9 ± 12.9 years. The duration of hemodialysis was 6.5 ± 5.9 years. Data regarding session length, dialyzer type, and dialysis mode were also collected. Although session length and dialysis did not change throughout the study period, the prevalence of high-flux dialyzer use increased from 42.5% to 50.7% over the study period (P < 0.05). Diabetes mellitus (n = 144, 40.6%), hypertension (n = 95, 26.8%), and glomerulonephritis (n = 73, 20.6%) were the major causes of ESRD.
Changes in serum concentrations of phosphorus, calcium, AP, and intact PTH
After applying our recommendations for 1 year, the final serum phosphorus concentration (4.9 ± 1.5 mg/dL) was not different from baseline (4.8 ± 1.5 mg/dL, P = 0.232). However, serum calcium concentration significantly increased from 8.9 ± 0.8 mg/dL to 9.2 ± 0.8 mg/dL (P < 0.001). The intact PTH level significantly decreased from 223 ± 235 pg/mL to 176 ± 218 pg/mL (P < 0.001). Consistent with this, serum AP decreased from 115 ± 88 U/L to 78 ± 45 U/L (P < 0.001) after the QI activities (Fig. 3).
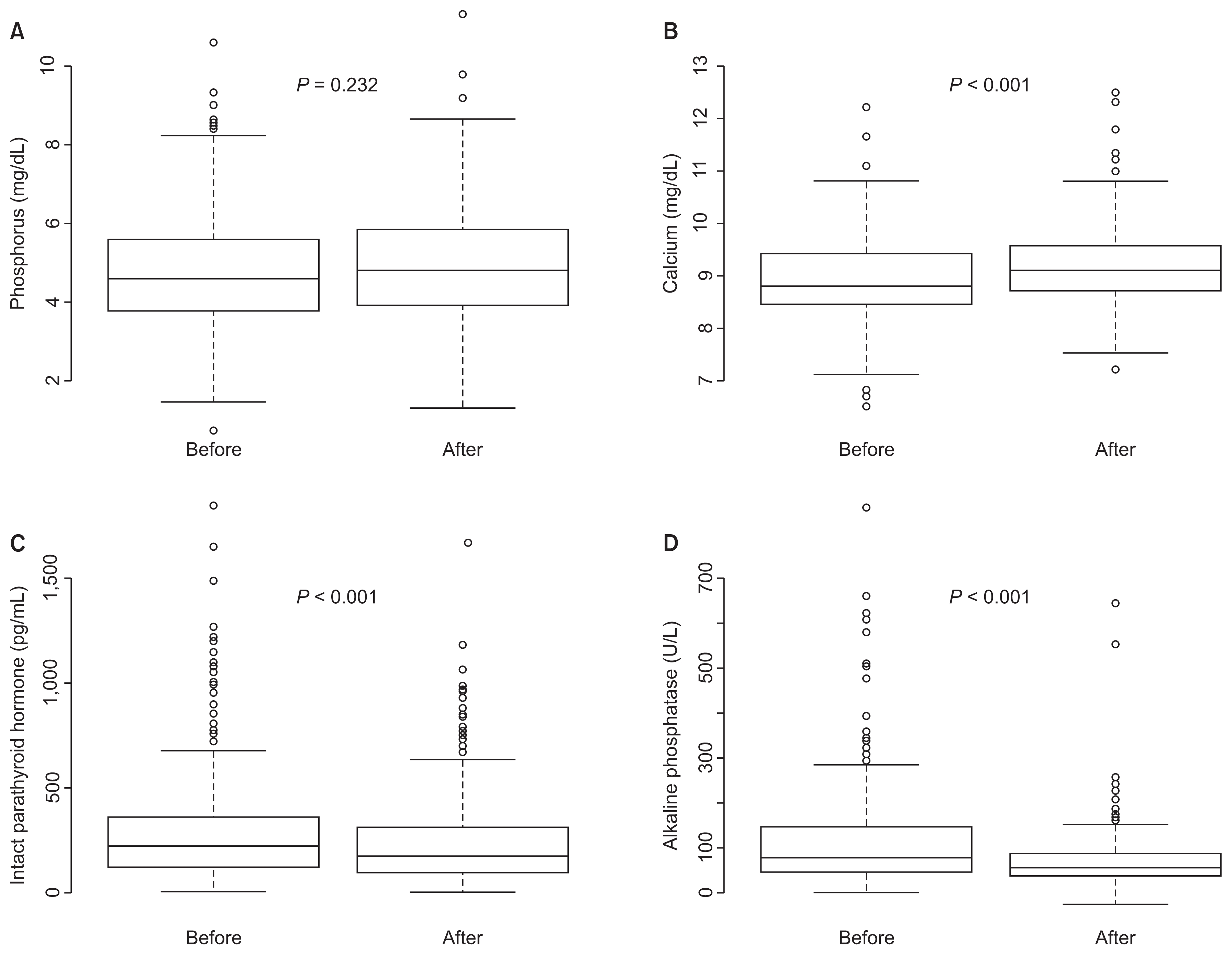
Comparisons of serum phosphorus (A), calcium (B), intact parathyroid hormone (C), and alkaline phosphatase (D) levels before and after implementing Korean Recommendations
Serum calcium concentration was significantly increased, and levels of serum intact parathyroid hormone and alkaline phosphatase were significantly decreased.
Changes in proportion of patients meeting the target
Next, we evaluated whether the proportion of patients meeting the target recommendations was improved by our strategy. Fig. 4 to 7 illustrate changes in the distribution of patients according to target ranges of serum phosphorus, calcium, intact PTH, and AP both before and after implementing this strategy.
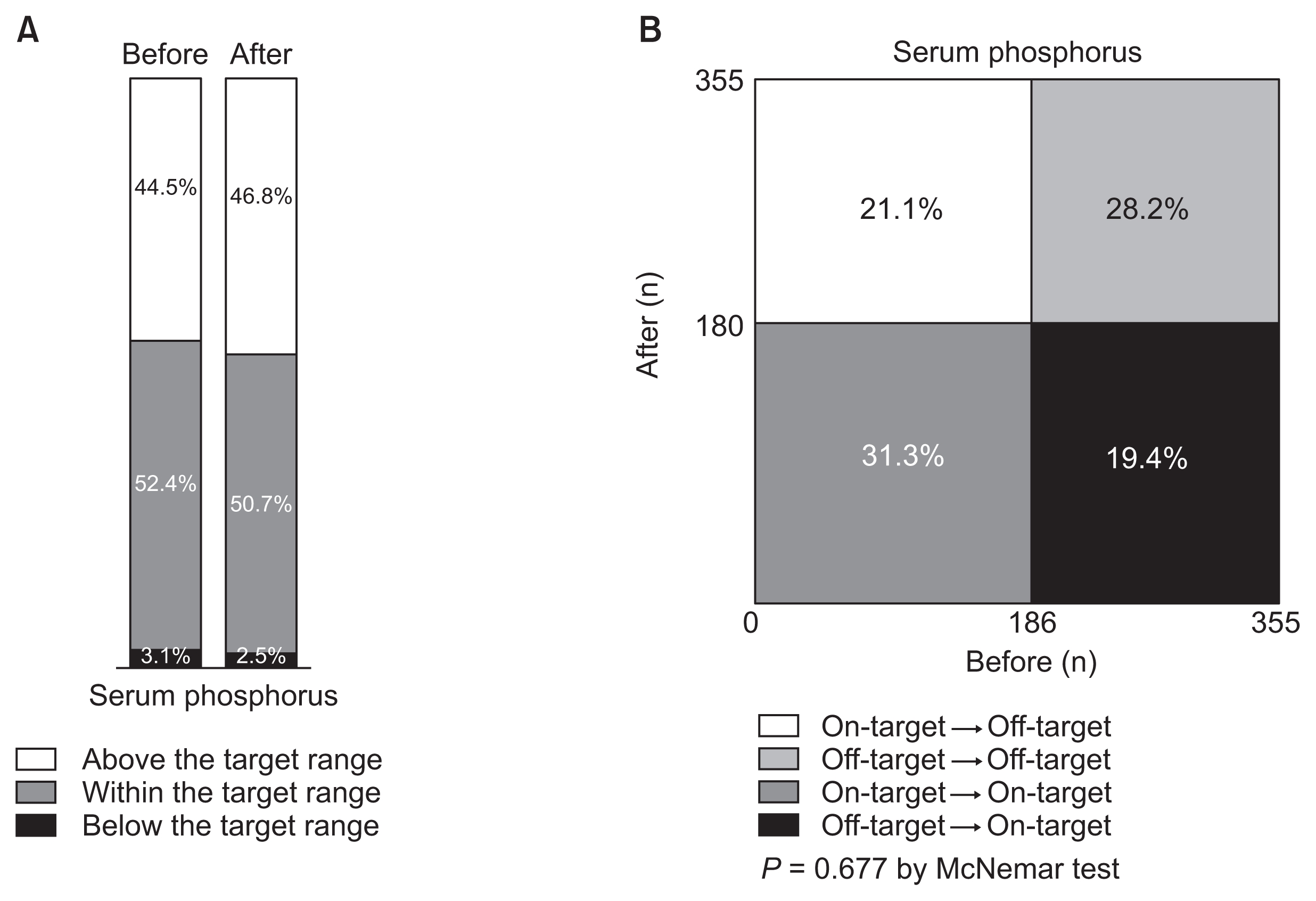
Changes in the proportion of patients within the target range (2.4–5.0 mg/dL) of serum phosphorus before and after implementing Korean Recommendations
Distributions are simply shown in A and statistically compared in B. The proportion of patients whose serum phosphorus was at the target was not significantly altered.
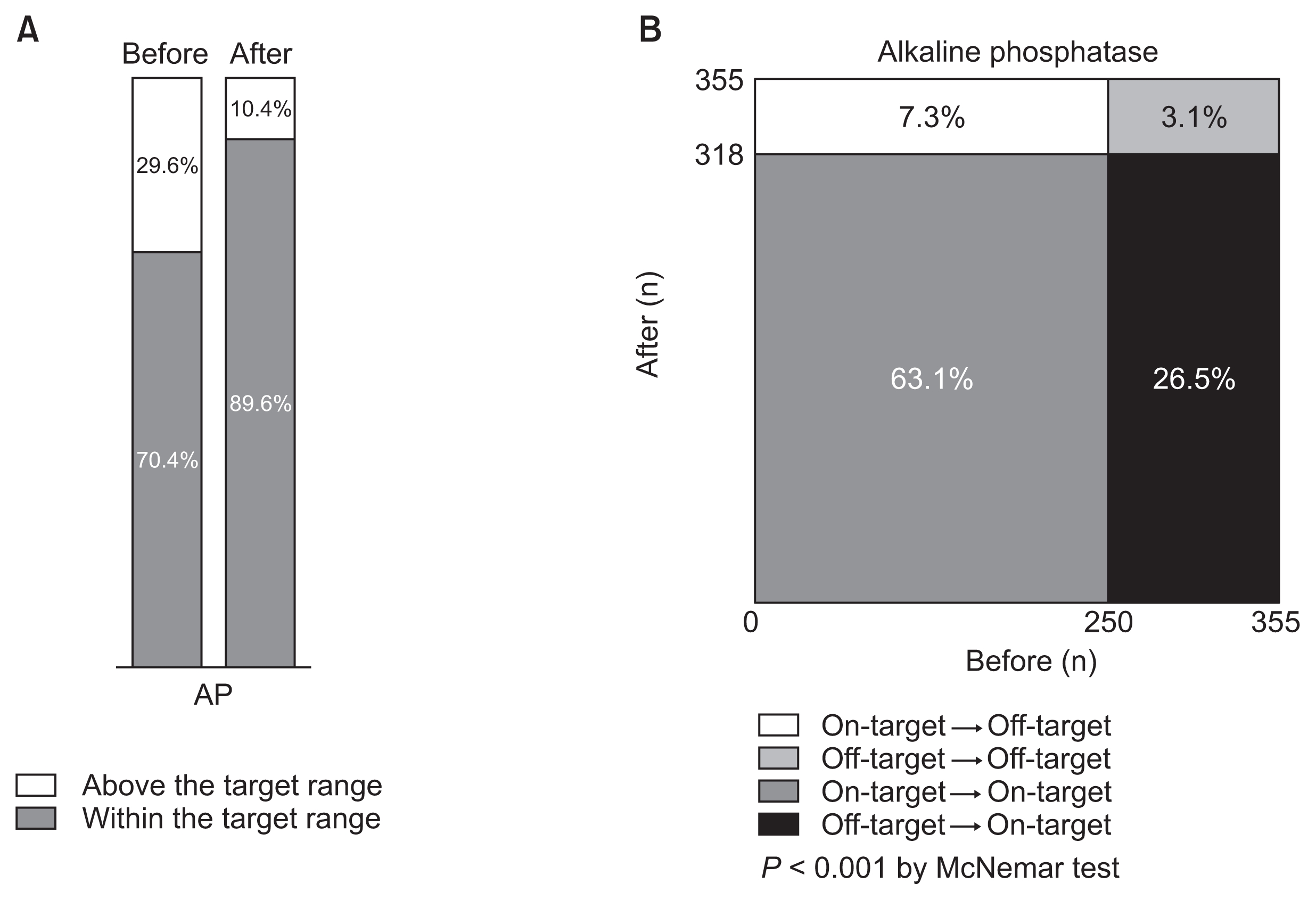
Changes in proportion of patients by the target range (< 120 U/L) of serum alkaline phosphatase (AP) before and after implementing Korean Recommendations
Distributions are simply shown in A, and statistically compared in B. The proportion of patients whose serum AP was at the target was significantly increased.
The target for serum phosphorus was 2.4 to 5.0 mg/dL [10]. Initially, 186 patients (52.4%) were within the target range of serum phosphorus (Fig. 4A). Similarly, 180 patients (50.7%) had the target level of serum phosphorus at the end of the study. Among 169 patients who were out of the target range at baseline, 69 patients (40.8%) were in the target range at the end of the study. However, 75 (40.3%) of 186 patients who were within the target range at baseline had moved out of the target range at the study end. As a result, the proportion of patients with serum phosphorus within the target range was not significantly changed (P = 0.677, Fig. 4B).
Our target for serum calcium was 8.4 to 9.6 mg/dL [10]. Initially, 215 patients (60.6%) were within the target range of serum calcium (Fig. 5A). Among these, only 138 patients (64.2%) had the target level of serum calcium at the end of the study. In addition, 85 patients (60.7%) entered the target range among 140 patients who were out of the target range at baseline. Finally, 223 patients (62.8%) had serum calcium within the target range when the study finished. Thus, the proportion of patients with serum calcium within the target range was not significantly affected (P = 0.582, Fig. 5B).

Changes in proportion of patients within target range (8.4–9.6 mg/dL) of serum calcium before and after implementing Korean Recommendations
Distributions are simply shown in A and statistically compared in B. The proportion of patients whose serum calcium was at the target was not significantly altered.
A total of 160 (45.1%) and 169 patients (47.6%) were within the target range of intact PTH (100–300 pg/mL) at baseline and at the end of the study, respectively (Fig. 6A). Only 95 patients (59.4%) remained within the target range of serum intact PTH among those who were initially at the target, whereas 74 (37.9%) of 195 patients who were out of the target at baseline entered the target range by the study end. Thus, the proportion of patients with intact PTH level within the target range was not significantly altered (P = 0.497, Fig. 6B). Conversely, the proportion of patients with serum intact PTH > 500 pg/mL significantly decreased from 51 (14.4%) to 34 (9.6%) after 1 year (P = 0.022).
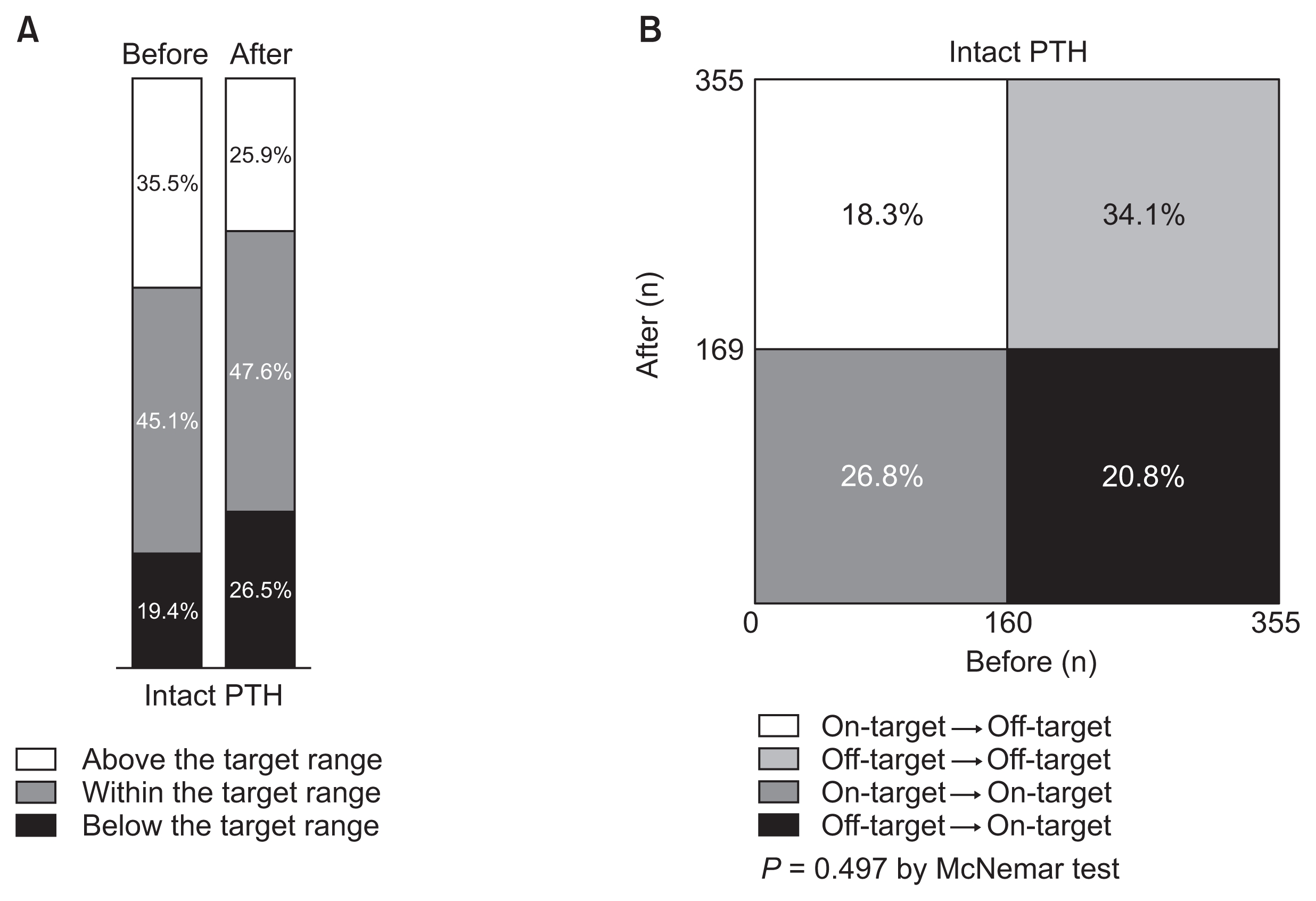
Changes in proportion of patients within target range (100—300 pg/mL) of serum intact parathyroid hormone (PTH) before and after implementing Korean Recommendations
Distributions are simply shown in A and statistically compared in B. The proportion of patients whose serum PTH was at the target was not significantly altered.
Serum AP results are the most notable. Initially, 250 patients (70.4%) were within the target range of serum AP (Fig. 7A). Most of these (237 patients) maintained their target level of serum AP at the end of the study. Among 105 patients who were out of the target at baseline, 81 patients entered the target range. Finally, 318 patients (89.6%) achieved the target level of serum AP at the study end. Thus, the proportion of patients with serum AP within the target range was significantly increased (P < 0.001, Fig. 7B).
Use of phosphate binders, vitamin D analogues, and cinacalcet
Table 2 shows the difference in medications between the beginning and end of implementing our domestic recommendations. Throughout the study period, more than half of the patients took calcium-based phosphate binders. The number of patients who used either calcium-based or calcium-free phosphate binders was not significantly changed. However, use of PTH-lowering agents, including vitamin D analogues (e.g., paricalcitol), and cinacalcet significantly increased during the study (P < 0.05).
Discussion
Management of CKD-MBD in patients on MHD is challenging because steady control of the parameters is difficult to achieve. Similar to a previous report from several years ago [10], only about half of our patients on MHD were within the target ranges of serum phosphorus, calcium, and intact PTH. However, some serum mineral parameters of CKD-MBD were affected by implementation of our local practice recommendations for patients on MHD. In particular, the serum AP level stabilized after adopting our strategy for 1 year to achieve target ranges for serum phosphorus, calcium, and intact PTH.
Treatment of hyperphosphatemia was the pivot of our recommendations for management of CKD-MBD. In addition to phosphate binders, dietary phosphorus restriction and adequate dialysis were implemented. However, the final serum phosphorus level was not different from the baseline in patients in this study. Interestingly, almost the same number of patients moved into and out of the target range of serum phosphorus during the study period. Thus, our efforts to improve control of hyperphosphatemia were not successful over a short period of time. Consistent with this, the prevalence of phosphate binders did not significantly change over the study period even though the use of calcium-based versus calcium-free phosphate binders was differentiated.
Albumin-corrected calcium concentrations were not obtained in this study because patients with malnutrition or intercurrent acute illness were excluded. Although the median value of serum calcium was increased by our strategy, the proportion of patients with serum calcium levels within the target range was not significantly altered. According to the reimbursement policy of our National Health Insurance program, calcium-based phosphate binders had to be initially prescribed. The increased prescription of both vitamin D receptor agonists and cinacalcet may explain the counterbalance in serum calcium by the end of our study. Data on vascular calcification were not included in this study.
The third mineral parameter in our recommendations is PTH. Our target for intact PTH is somewhat low compared with the KDIGO guidelines [1], but similar to the KDOQI and Japanese guidelines [3,5]. Consistent with the change in serum calcium, the median value of serum intact PTH was decreased in this study. Suppression of PTH and AP was associated with the use of both vitamin D analogues and cinacalcet. However, our attempt to improve control of secondary hyperparathyroidism was not successful because the proportion of patients with serum intact PTH levels within the target range did not significantly increase.
In contrast, the changes in serum AP were remarkable. Our strategy to manage patients with CKD-MBD was associated with a decrease in the median value of serum AP after a short period of time. Furthermore, the number of patients with serum AP levels within the target range was significantly increased by implementation of our domestic practice recommendations. We believe that use of vitamin D receptor agonists and cinacalcet was successful in modifying high turnover bone disease. Among routine clinical and biochemical markers, serum AP is a sensitive marker of bone mineral density in patients on MHD [11].
We did not previously include any suggestions on the target level of serum AP in patients on MHD because serum total AP may be seen as an adjunct to PTH measurement with no clearly defined management targets [12]. However, the recently revised 2017 KDIGO CKD-MBD guidelines recommend monitoring total or bone-specific serum AP activity in patients with advanced CKD [2]. Although bone formation rate was better correlated with plasma bone-specific AP levels than with either plasma total AP or intact PTH concentrations [13,14], serum total AP was used because of its feasibility for routine monitoring in patients on MHD. A large cohort study showed that increased serum total AP > 120 U/L was associated with mortality among patients on MHD [15]. Unlike the U- or J-shaped association of serum phosphorus or PTH with mortality in patients on chronic dialysis, serum total AP level had a more linear association with mortality. Other studies also showed that only higher serum AP concentrations were associated with increased mortality [16,17]. Thus, we defined our target for serum AP as ≤ 120 U/L.
There are several limitations to this study. There were a small number of patients, the follow-up period was short, and parameters related to hard outcomes were not obtained. Among the 7 dialysis units that participated in this study, only a few centers previously had their own CKD-MBD treatment protocols. Most centers complied with the national health insurance guidelines and adopted international guidelines such as KDOQI and KDIGO. Because the general principles in treating CKD-MBD in patients on MHD were not substantially changed by our new strategy, strict adherence to a certain recommendation or overcoming the physician’s inertia is very important to achieve quality care. Within a certain guideline, individualized approaches would enhance achievement as well. Nonetheless, we showed that our local adaptation for managing CKD-MBD in patients on MHD was feasible and can be applied as a QI strategy. To the best of our knowledge, few previous studies have reported adaptation of global clinical practice guidelines for QI of local practices.
In conclusion, we carried out a simplified medication adjustment over 1 year to achieve certain targets of serum phosphorus, calcium, and intact PTH in patients on MHD. Although serum phosphorus, calcium, and PTH levels were not effectively controlled over a short period of time, serum AP level improved in our patients. This stabilization of serum AP activity in patients with CKD-MBD will ameliorate high turnover bone disease and possibly improve mortality. Long-term follow-up evaluations are necessary to determine whether our modifications affect uremic bone disease and cardiovascular calcifications.
Acknowledgments
This study was funded by AbbVie Korea.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.