Effects of oxidative stress on endothelial modulation of contractions in aorta from renal hypertensive rats
Article information
Abstract
Background
Endothelial dysfunction is linked to exaggerated production of superoxide anions. This study was conducted to examine the effects of oxidative stress on endothelial modulation of contractions in chronic two-kidney, one-clip (2K1C) renal hypertensive rats.
Methods
The 2K1C hypertension was induced by clipping the left renal artery; age-matched rats receiving sham treatment served as controls. Thoracic aortae were isolated and mounted in tissue baths for measurement of isometric tension.
Results
Norepinephrine-induced contraction was augmented by the removal of the endothelium, which was more pronounced in sham rats than in 2K1C rats. Nω-nitro-L-arginine methyl ester, an inhibitor of nitric oxide production, had a similar augmenting effect. Vitamin C inhibited the contraction in aortic rings with intact endothelium from 2K1C rats but not from sham rats. The contraction was also suppressed by treatment with diphenyleneiodonium or apocynin, inhibitors of nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide phosphate (NADH/NADPH) oxidase, in the aortae with intact endothelium from 2K1C rats but not in those from sham rats. Superoxide anions generated by xanthine oxidase/hypoxanthine enhanced the contraction in the aortae with intact endothelium from sham rats, but had no effect in 2K1C rats. Enhanced contractile responses to norepinephrine by xanthine oxidase/hypoxanthine in sham rats were reversed by vitamin C.
Conclusion
These results suggest that the effect on endothelial modulation of endothelium-derived nitric oxide is impaired in 2K1C hypertension. The impairment is, at least in part, related to increased production of superoxide anions by NADH/NADPH oxidase.
Introduction
Initially regarded as an inert lining between blood and blood vessels, the vascular endothelium has a critical role in the maintenance of vascular tone and blood pressure by generating various vasoactive substances [1]. In physiological states, the endothelium releases both relaxing and contracting factors; a balance between these two factors contributes to the local regulation of vascular tone [2]. It has been known that hypertension is characterized by endothelial dysfunction in large conduit and small resistance arteries [3], [4]. Endothelium-dependent vasodilation is indeed impaired in a number of experimental models, including two-kidney, one-clip (2K1C) hypertension [5], [6], [7]. Reduced production of endothelial relaxing factors or increased production of endothelial contractile substances may be responsible for the depressed endothelium-dependent relaxation in hypertension [8].
In addition to the direct relaxing or contracting effects of the endothelium, it can also modulate the effects of vasoconstrictor substances. The important role of the vascular endothelium in the response of isolated vascular segments to several vasoconstrictors, including norepinephrine, is widely recognized [6], [9]. Constrictors apparently interact with the endothelium and induce the release of relaxing factors, which then have an inhibitory effect on vascular smooth muscle tone [6], [10]. In our previous study, we demonstrated that the endothelium plays an inhibitory role against contractile responses to norepinephrine by releasing nitric oxide (NO), and the endothelial inhibition is attenuated in 2K1C hypertension [11]. Nevertheless, the mechanisms underlying the impaired endothelial modulation in hypertension remain to be established.
An endothelial dysfunction is known to be linked to the exaggerated production of superoxide anions. It has been proposed that increased oxidative inactivation of NO caused by an excess of superoxide anions is responsible for reducing the bioavailability of NO, which is in part related to endothelial dysfunction in hypertension [12], [13]. Miyagawa et al [14] reported that vascular oxidative stress could contribute to an altered circulation by impairment of endothelial modulation of vascular contractions in spontaneously hypertensive rats. In addition, an increase in oxidative stress systemically plays a major role in the maintenance of high arterial blood pressure and sympathetic drive in renal hypertension [15]. We also observed that hydrogen peroxide may contribute in part to the altered vascular relaxation in 2K1C hypertensive rats [16].
The current study was undertaken to determine whether oxidative stress is involved in the impaired endothelial modulation of vascular contractions in 2K1C renal hypertension.
Methods
Induction of 2K1C renal hypertension
Male Sprague-Dawley rats (160–180 g) were anesthetized with sodium thiopental (40 mg/kg, IP). The left posterior side of the animal was shaved and sterilized with 70% ethanol. An incision of approximately 2 cm in length was made through the skin and muscles just below the ribs. The left kidney was then exposed and retracted in order to expose the renal artery, and a silver clip (internal diameter 0.2 mm) was applied to the exposed renal artery. The clip was then turned such that the slit opening was facing the abdomen. The contralateral kidney was left intact. The muscles and skin were then sutured, and the animal was left to recover from anesthesia. Control rats received a sham treatment, which involved exactly the same procedure except that no clip was placed. Postoperatively, all animals were fed normal chow and given tap water. They were operated at 10 weeks after clipping, because endothelial dysfunction is associated with the duration of hypertension [17]. Hypertensive rats were selected based on systolic blood pressure measured in a conscious state using the tail-cuff method and a piezoelectric pneumotaxic pulse transducer.
Tissue preparation
The thoracic aorta between the aortic arch and diaphragm was removed rapidly and placed in a physiological salt solution of the following composition: NaCl 118.3mM, KCl 4.7mM, NaHCO3 25mM, MgCl2 1.2mM, KH2PO4 1.2mM, CaCl2 2.5mM, and glucose 11.1mM. Vessels were cleaned of adhering tissue and cut into rings (2 mm wide) under a dissection microscope. Care was taken not to stretch the artery or damage the luminal surface. In some preparations, the endothelium was removed by gentle rubbing of the intimal surface with a moist cotton swab. Successful removal of endothelial cells from aortic rings was confirmed by the inability of acetylcholine to induce relaxation.
The aortic rings were suspended using two triangular-shaped stainless-steel holders in the vessel lumen in organ chambers containing 15 mL of physiological salt solution maintained at 37°C and aerated with 95% O2 and 5% CO2. To measure isometric tension development, one of the holders was fixed at the bottom of the chambers and the other was connected to a force displacement transducer (FTO3; Grass Technologies, Warwick, RI, USA). Prior to initiating specific experimental protocols, aortic rings were stretched to the point of their optimal length–tension relationship, which was determined to be 2 g by similar preliminary experiments involving repeated exposure to 60mM KCl solution (obtained by equimolar replacement of NaCl by KCl in physiological solution), and allowed to equilibrate for 90 minutes. After equilibration, rings were maximally contracted by the 60mM KCl solution to test their contractile capacity.
Protocols
During the first set of experiments, contractile responses to norepinephrine (10−10–10−5M) were determined in rings with or without the endothelium. To obtain α-adrenoceptor-mediated responses to norepinephrine, aortic rings were pretreated with a β-adrenoceptor antagonist timolol (3×10−7M). To confirm the role of NO in the endothelium-dependent modulation of vascular contraction, concentration–response curves of norepinephrine were obtained in the presence of Nω-nitro-L-arginine methyl ester (L-NAME; 10−4M), which inhibits the endogenous production of NO from L-arginine.
In the second set of experiments, to verify the role of oxidative stress and the involvement of nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide phosphate (NADH/NADPH) oxidase in endothelial dysfunction in 2K1C rats, effects of vitamin C (10−4M) or inhibitors of NADH/NADPH oxidase, diphenyleneiodonium (DPI; 10−5M), or apocynin (3×10−5M) on the contractile responses to norepinephrine [14] were determined. In order to assess the potential role of other enzymatic sources of superoxide anions, we recorded the concentration–response to norepinephrine of aortic rings pretreated with allopurinol (3×10−4M), a xanthine oxidase inhibitor.
In the third set of experiments, using the hypoxanthine–xanthine oxidase system, which generates superoxide anions and hydrogen peroxides [18], we examined the effects of superoxide anions on the contractile response to norepinephrine. Concentration–response curves of norepinephrine in the absence or presence of hypoxanthine (2×10−4M)/xanthine oxidase (20 U/L) plus catalase (1,000 U/mL) were plotted.
Drugs
The drugs used in this study were acetylcholine chloride, apocynin (Calbiochem, Los Angeles, CA, USA), catalase, DPI, hypoxanthine, L-norepinephrine bitartrate, L-NAME, timolol maleate, vitamin C (L-ascorbic acid), and xanthine oxidase. They were purchased from Sigma Chemical Co. (St Louis, MO, USA) unless otherwise stated. All drugs were administered in volumes not exceeding 0.5% of the bath volume.
Statistical analysis
Contractile responses are percentages of contractions elicited by 60mM KCl. Values presented in the figures are means and standard errors of the means, and the numbers of aortic rings tested are provided in parentheses. Statistical comparisons were performed using the unpaired t test or analysis of variance with repeated measurements and Fischer’s post hoc test. Differences were considered significant for P<0.05.
Results
Ten weeks after surgery, the systolic blood pressure was 136±3 mmHg (n=38) and 190±4 mmHg (n=40) in sham-clipped control and 2K1C hypertensive rats, respectively (P<0.05). The magnitude of KCl (60mM)-induced isometric tension development was comparable in the two groups (1.41±0.10 g in control and 1.49±0.13 g in 2K1C rats).
In aortic rings from sham-clipped control and 2K1C rats, norepinephrine induced contraction in a concentration-dependent manner. The contractile response to norepinephrine was augmented in 2K1C rats compared to that in sham rats. Norepinephrine-induced contraction was enhanced by the removal of the endothelium in sham rats, but not in 2K1C rats. L-NAME treatment had an effect similar to that of endothelium removal (Fig. 1).

Concentration–response curves of norepinephrine in aortic rings with (+) or without (−) endothelium from sham-operated (A) and 2K1C hypertensive (B) rats. Results obtained from rings with endothelium in the presence of L-NAME (10−4M) are also shown. Results are representative of six to eight experiments.
⁎, †P<0.05, compared with corresponding +Endo values.
Endo, endothelium; L-NAME, Nω-nitro-L-arginine methyl ester; 2K1C, two-kidney, one clip.
To determine whether the impaired endothelial inhibition of norepinephrine-induced contraction is related to oxidative stress, effects of vitamin C on norepinephrine-induced contraction were examined in aortic rings with intact endothelium. Vitamin C inhibited the contractile response to norepinephrine in 2K1C rats but not in sham controls (Fig. 2).
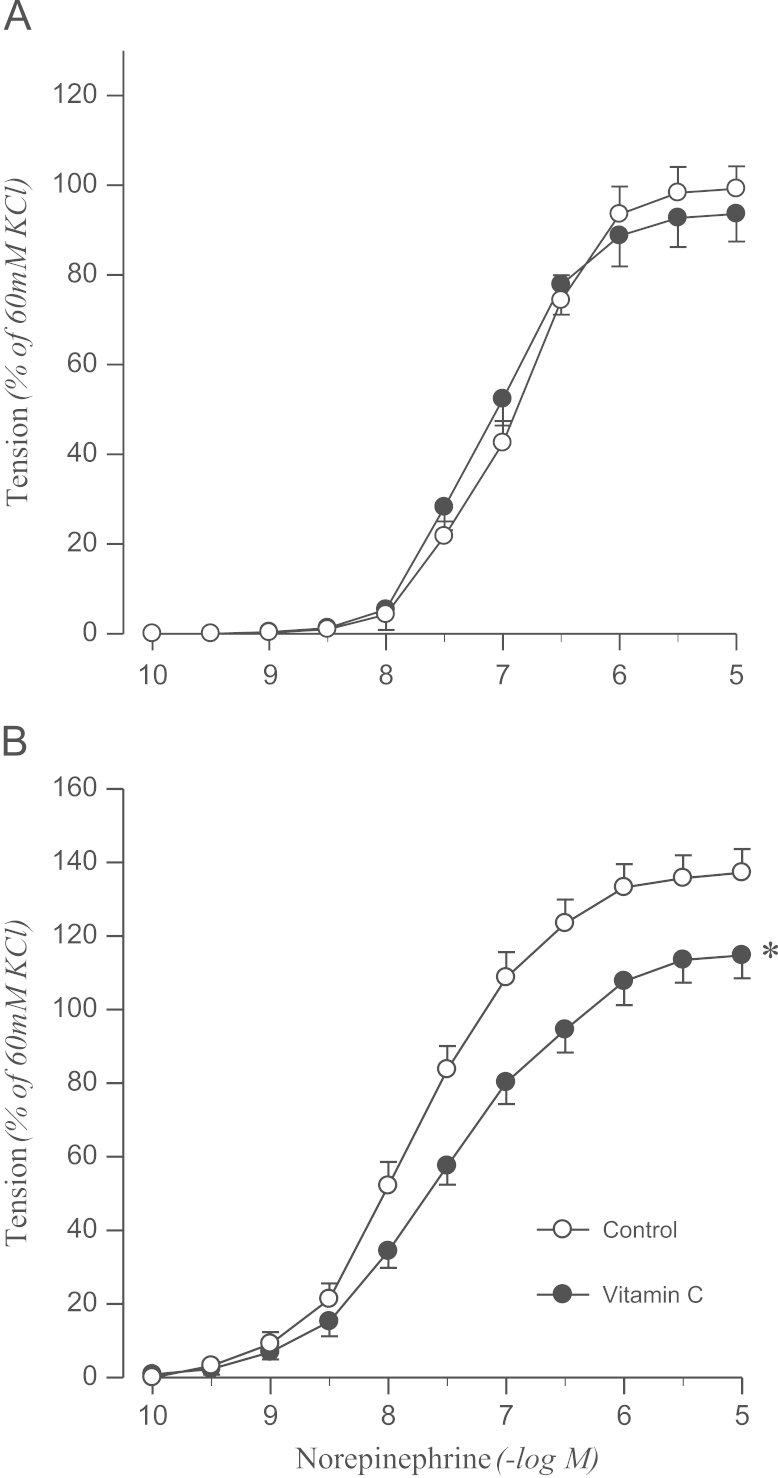
Effects of vitamin C on concentration–response to norepinephrine in aortic rings with endothelium from sham-operated (A) and 2K1C hypertensive (B) rats. Results are representative of six to eight experiments.
⁎P<0.05, compared with corresponding control values.
2K1C, two-kidney, one clip.
To confirm the origin of oxidative stress, the effects of DPI or apocynin on norepinephrine-induced contraction were examined. DPI attenuated the contractile response to norepinephrine in the aortic rings from 2K1C rats, but not in sham rats (Fig. 3). Norepinephrine-induced contraction was also attenuated by pretreating aortic rings with apocynin in 2K1C rats but not in controls (Fig. 4).
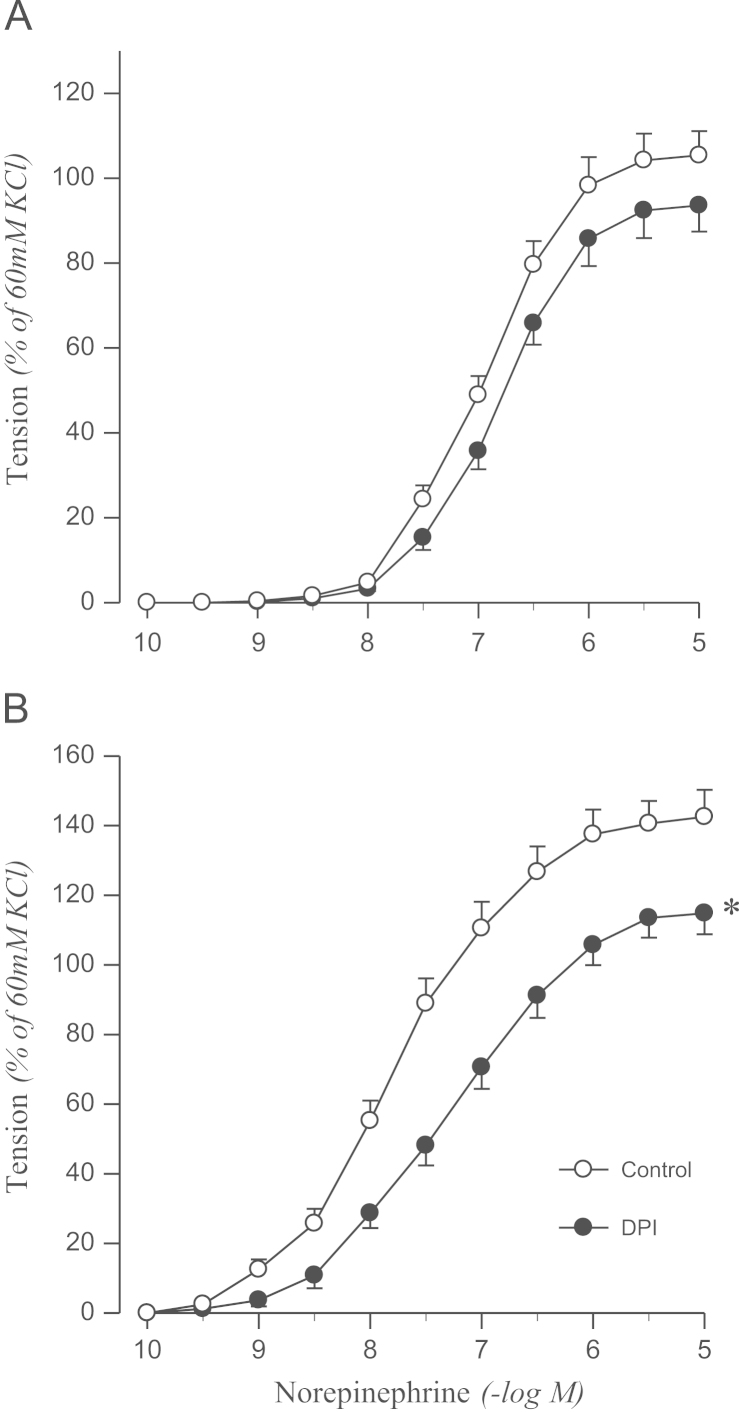
Effects of DPI on concentration–response to norepinephrine in aortic rings with endothelium from sham-operated (A) and 2K1C hypertensive (B) rats. Results are representative of six to eight experiments.
⁎P<0.05, compared with corresponding control values.
DPI, diphenyleneiodonium; 2K1C, two-kidney, one clip.
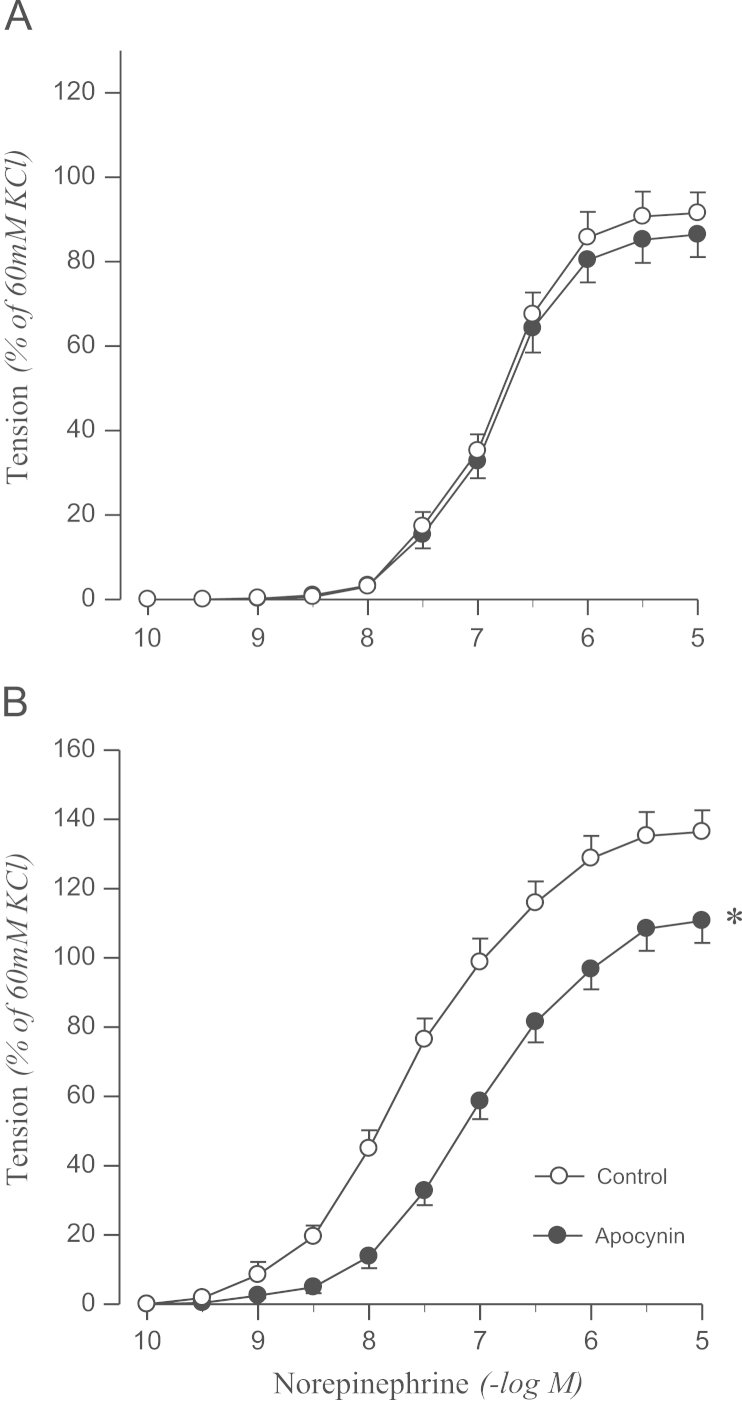
Effects of apocynin on concentration–response to norepinephrine in aortic rings with endothelium from sham-operated (A) and 2K1C hypertensive (B) rats. Results are representative of six to eight experiments.
⁎P<0.05, compared with corresponding control values.
2K1C, two-kidney, one clip.
By contrast, allopurinol, a xanthine oxidase inhibitor, affected the contractile response to norepinephrine neither in 2K1C rats nor in sham rats (data not shown).
Norepinephrine-induced contraction was enhanced by treatment with xanthine oxidase in the presence of hypoxanthine in aortic rings from sham rats, which was abolished by vitamin C. However, the hypoxanthine–xanthine oxidase system had no effect on the contractile response to norepinephrine in 2K1C rats (Fig. 5).
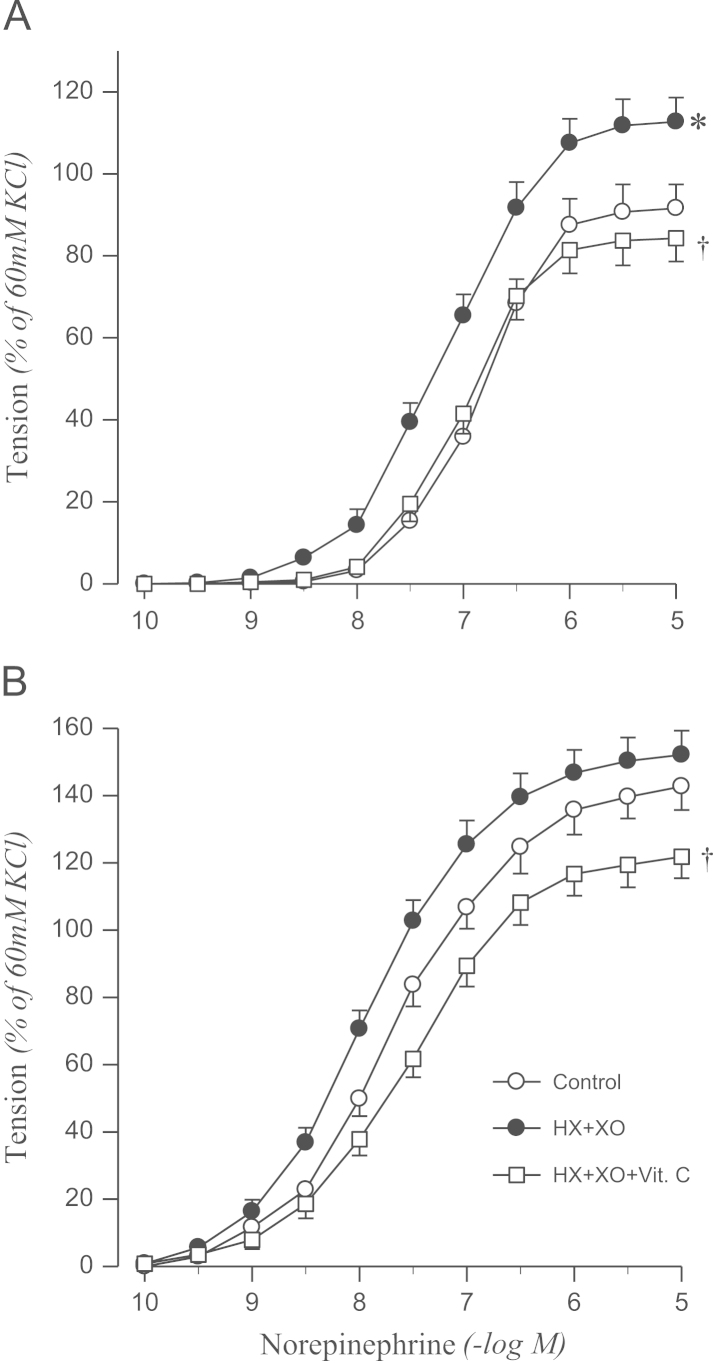
Effects of HX+XO and HX+XO+Vit. C in the presence of catalase on concentration–response to norepinephrine in aortic rings with endothelium from sham-operated (A) and 2K1C hypertensive (B) rats. Results are representative of six to eight experiments.
⁎P<0.05, compared with corresponding control values.
†P<0.05, compared with corresponding HX + XO values.
HX, hypoxanthine; Vit. C, vitamin C; XO, xanthine oxidase; 2K1C, two-kidney, one clip.
Discussion
The vascular response to norepinephrine was enhanced in 2K1C hypertension, as was in our previous studies [10], [11]. The augmented contraction in hypertension has been attributed to augmented phosphoinositide hydrolysis, greater release of intracellular Ca2+ from a cellular pool, increased activation of protein kinase C, or alterations in the number and affinity of inositol trisphosphate receptors on the endoplasmic reticulum of vascular smooth muscle [19]. Many of these processes may also be responsible for the increased sensitivity of vascular response to norepinephrine in 2K1C hypertension.
In the present study, endothelium removal and L-NAME treatment had similar effects on norepinephrine-induced contraction, which suggest that NO plays a critical role in endothelial modulation of norepinephrine-induced contraction. As was found in our previous study [11], the inhibitory effect of the endothelium on the norepinephrine-induced contraction was also attenuated in 2K1C rats in the present study. The endothelial dysfunction may be an impaired relaxation or an exaggerated contractile response. In 2K1C hypertensive rats, the impaired endothelial modulation of vascular contractions induced by norepinephrine may be related to a decreased production of NO, an increase in inactivation of NO, and/or altered NO-cyclic guanosine monophosphate (cGMP) signaling. We previously showed that relaxations induced by sodium nitroprusside are similar in aortic rings from 2K1C and sham control rats [11]. Based on the abovementioned findings, NO-cGMP signaling appears to be preserved in 2K1C hypertension. In addition, Miyagawa et al [14] demonstrated that NO levels were not altered, but rather the function of the endothelium was impaired. We also previously found that plasma concentrations of NO were comparable between 2K1C and sham rats [20]. Thus, it appears that the production of NO is not altered in hypertension, but its inactivation is enhanced.
Previous studies have demonstrated that the production of superoxide anions by the vascular wall is enhanced in various models of hypertension, including 2K1C rats [14], [15], [21], [22]. In the present study, contractile responses to norepinephrine were inhibited significantly by pretreatment with vitamin C, an antioxidant, in the aortic rings from 2K1C rats, but not in those from sham control rats. It has been reported that impaired endothelium-dependent relaxation induced by acetylcholine in hypertension is improved by vitamin C, which acts as a free radical scavenger [23]. Therefore, the production of reactive oxygen species may play a role in the impaired endothelial modulation of vascular contractions in 2K1C hypertension, although vitamin C could either increase endothelial NO synthase (eNOS) activity or reduce the production of superoxide anions by inhibiting NADH/NADPH oxidase [24]. An enhanced production of superoxide anions may result in inactivation of NO and generation of peroxynitrite [15]. The resulting decrease in availability of NO may have a role in the impaired endothelial modulation of arterial contractions.
Superoxide anions have several possible sources, including NADH/NADPH oxidase, eNOS, and xanthine oxidase. It has been suggested that the generation of superoxide anions within the vascular wall is responsible for NADH/NADPH oxidase [14], and its involvement in the production of superoxide anions in the arteries from 2K1C hypertensive rats has also been reported [22]. As expected, DPI, a NADH/NADPH oxidase inhibitor, attenuated the norepinephrine-induced contraction in aortic rings from 2K1C rats but had no effect in those from sham rats in the present study. These results indicate that the production of superoxide anions in hypertensive vascular tissue is related to a NADH/NADPH oxidase-dependent mechanism in norepinephrine-induced contractions. Furthermore, another specific inhibitor of NADH/NADPH oxidase [14], apocynin, which is an endothelium-dependent as well as endothelium-independent relaxant [25], also reversed the impaired endothelial modulation in 2K1C rats. Therefore, NADH/NADPH oxidase appears to be the most plausible source of excessive production of superoxide anions in 2K1C hypertension, although DPI may also inhibit eNOS. To our knowledge, this is the first study showing that the source of superoxide production for the impaired endothelial modulation in chronic renal hypertension.
By contrast, allopurinol, a xanthine oxidase inhibitor, did not affect norepinephrine-induced contraction in either sham or 2K1C rats, as has been previously shown in genetically hypertensive rats [14]. In addition, nitrite has been reported to exert antihypertensive effects in 2K1C hypertensive rats, which may be related to its antioxidant properties resulting from the inhibition of vascular NADPH oxidase activity but not from the inhibition of xanthine oxidase activity [26]. Therefore, xanthine oxidase is unlikely to be involved in the production of superoxide anions under the experimental conditions of this study.
Renovascular hypertension caused by renal artery stenosis stimulates the renin–angiotensin system, thereby elevating circulating angiotensin II (Ang II) levels. However, despite the fact that the 2K1C model has been investigated extensively, the exact mechanisms responsible for the maintenance of hypertension in this model remain unclear. One hypothesis is that an increase in Ang II can activate NADH/NADPH oxidase and produce superoxide anions, thus decreasing the bioavailability of NO and increasing vasoconstriction, in addition to increasing the sympathetic activity [15]. Ang II has been shown to stimulate the generation of superoxide anion radicals in cultured vascular smooth muscle cells [27]. Furthermore, the expression of NADH/NADPH oxidase has been reported to be upregulated in 2K1C hypertension [15]. Taken together, it cannot be ruled out that Ang II may affect the production of superoxide anions in 2K1C hypertension, although we did not determine the concentration of Ang II.
Oxidant stress conditions can be created in isolated aortic rings by the generation of superoxide anions via the hypoxanthine–xanthine oxidase system [28]. However, these conditions are complicated by the fact that superoxide-generating systems also increase other reactive oxygen species such as hydrogen peroxide, hydroxyl radicals, and peroxynitrite, which can damage the endothelium and impair endothelial modulation [29]. We wished to examine specifically the interaction between NO and superoxide anions. Therefore, catalase was included in our experiment [14]. In the present study, the hypoxanthine–xanthine oxidase system enhanced the norepinephrine-induced contraction in aortic rings from sham-clipped rats in the presence of catalase. The augmented contractile response to norepinephrine in controls was abolished by the addition of vitamin C, suggesting that the observed impaired endothelial modulation of vascular contractions is due to increased superoxide anions. In 2K1C rats, however, norepinephrine-induced contraction was not altered by treatment with the hypoxanthine–xanthine oxidase system. The possible explanations for these observations may be that the released NO is inactivated mostly by endogenous superoxide anions derived from NADH/NADPH oxidase in hypertensive vascular tissues, and that the additional inhibitory effects against NO may not have been evoked by the generation of exogenous superoxide anions.
In summary, endothelial modulation of vascular contractions is impaired in the aortae of 2K1C hypertensive rats. The impairment may, at least in part, be due to an increased production of superoxide anions by NADH/NADPH oxidase. Vascular oxidative stress may be involved in the endothelial dysfunction in chronic 2K1C renal hypertension.
Conflicts of interest
The authors declare no conflicts of interest.
References
Acknowledgments
This study was supported by research funds from Chosun University, Gwangju, Korea, 2012.