| Kidney Res Clin Pract > Volume 42(4); 2023 > Article |
|
Abstract
Background
Methods
Results
Supplementary Materials
Notes
Funding
This research was funded by the Chung-Kang Branch of the Cheng Ching General Hospital Research Fund (CH11100268A).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authorsâ contributions
Conceptualization, Funding acquisition: CPL, THY, SHK, YHH
Data curation, Methodology: YSC, CYK
Formal analysis: YSC
Investigation: YSC, HLC, SHK, YHH
Project administration: THY, CYK, HLC, SHK
Writingâoriginal draft: SHK, YHH
Writingâreview & editing: CPL, SHK, YHH
All authors read and approved the final manuscript.
Figure 1.
Melatonin (Mel) cooperatively promotes the antitumoral activity of pazopanib (Paz) against human 786-O and A-498 renal cell carcinoma cells.
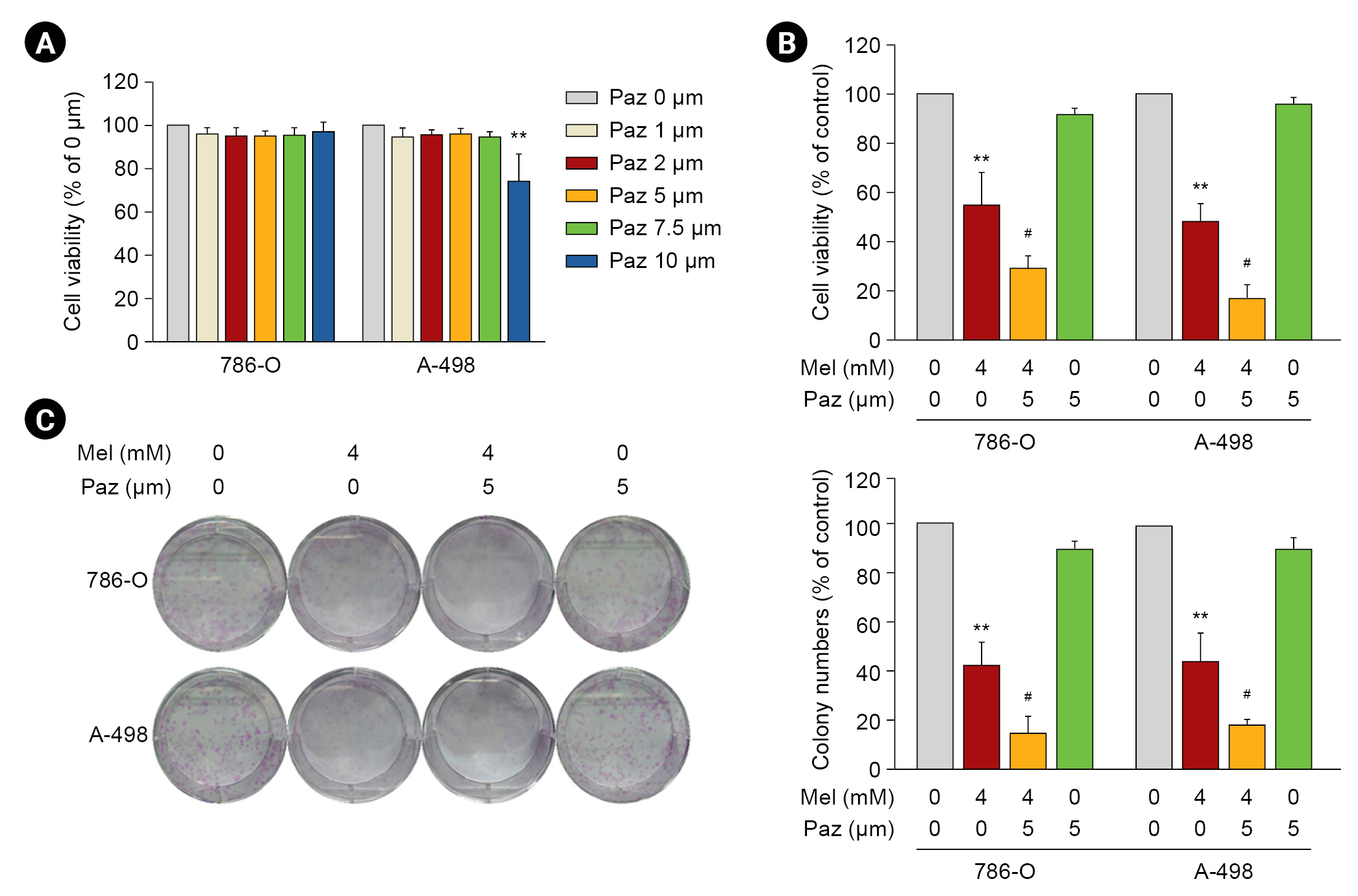
Figure 2.
Melatonin (Mel) combined with pazopanib (Paz) synergistically promotes caspase-dependent apoptosis in human 786-O and A-498 renal cell carcinoma cells.
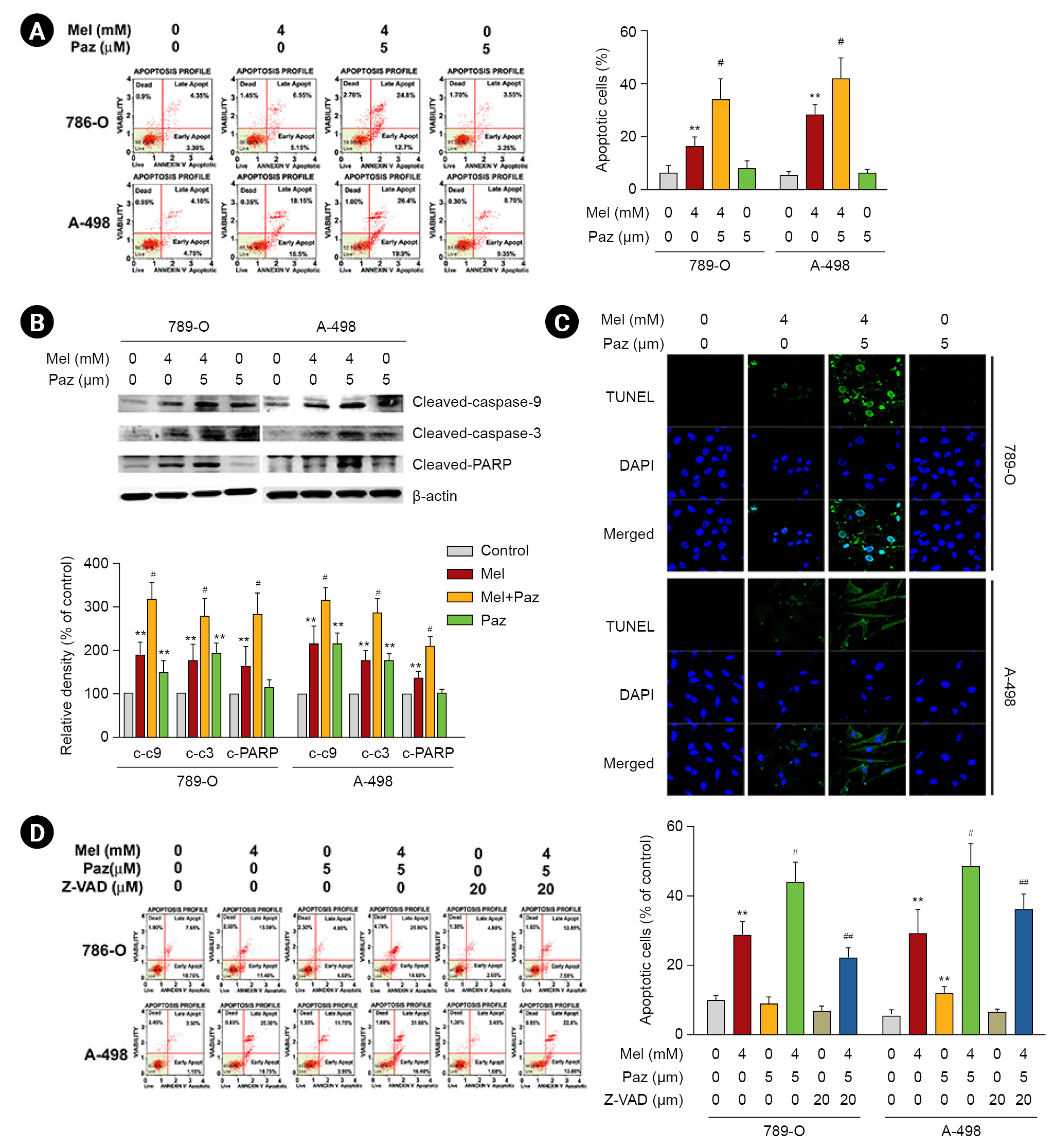
Figure 3.
Melatonin (Mel) combined with pazopanib (Paz) potentiates mitochondrial apoptosis in human 786-O and A-498 renal cell carcinoma cells.

Figure 4.
Melatonin (Mel) combined with pazopanib (Paz) induces autophagy-mediated apoptosis in human 786-O and A-498 renal cell carcinoma cells.
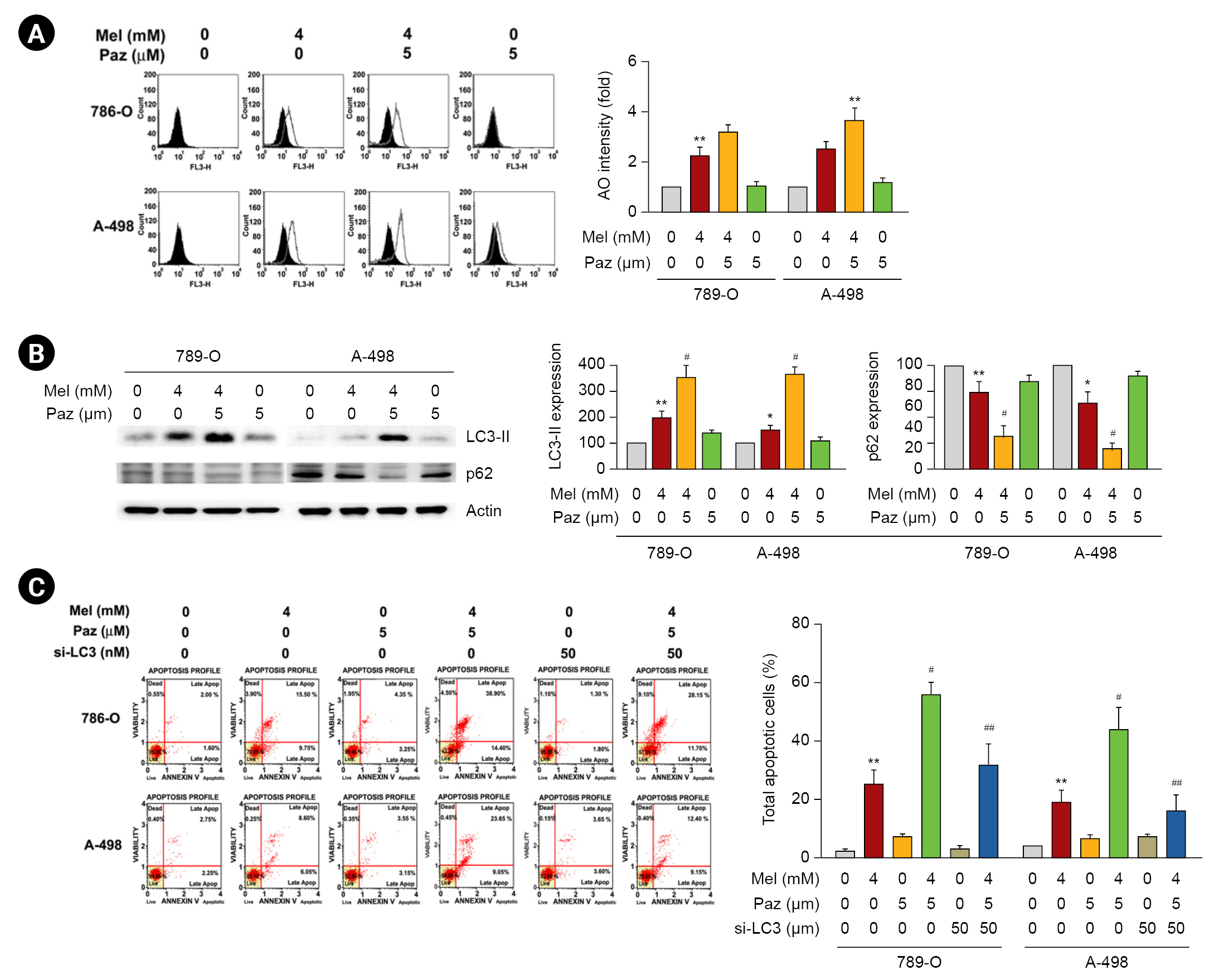
Figure 5.
Involvement of p38MAPK in the autophagy-mediated apoptotic cascade induced in human 786-O and A-498 renal cell carcinoma cells by melatonin (Mel) combined with pazopanib (Paz).
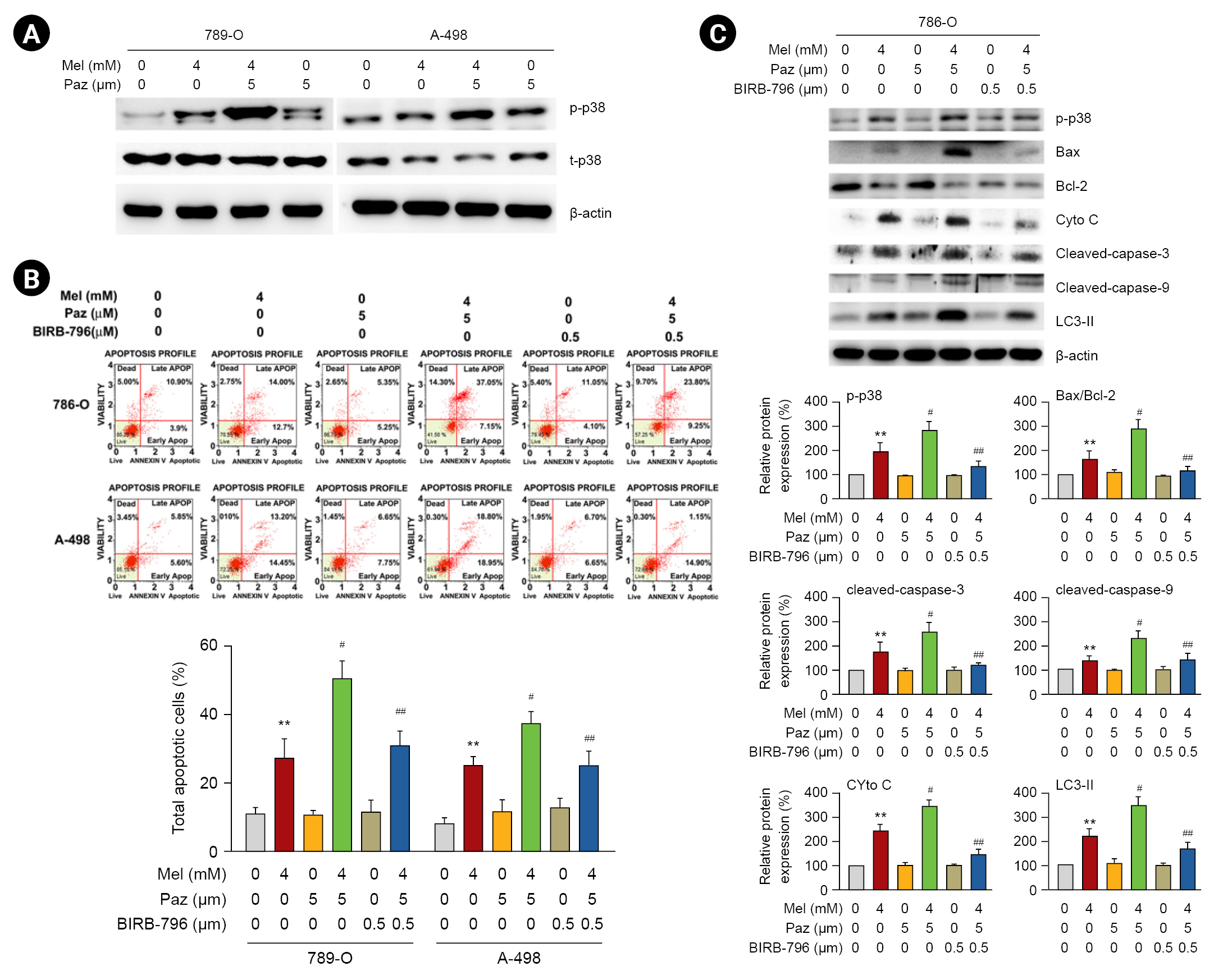
Figure 6.
Melatonin (Mel) combined with pazopanib (Paz) reduces in vivo tumor growth, and Mel/Paz interact with LC3-II.
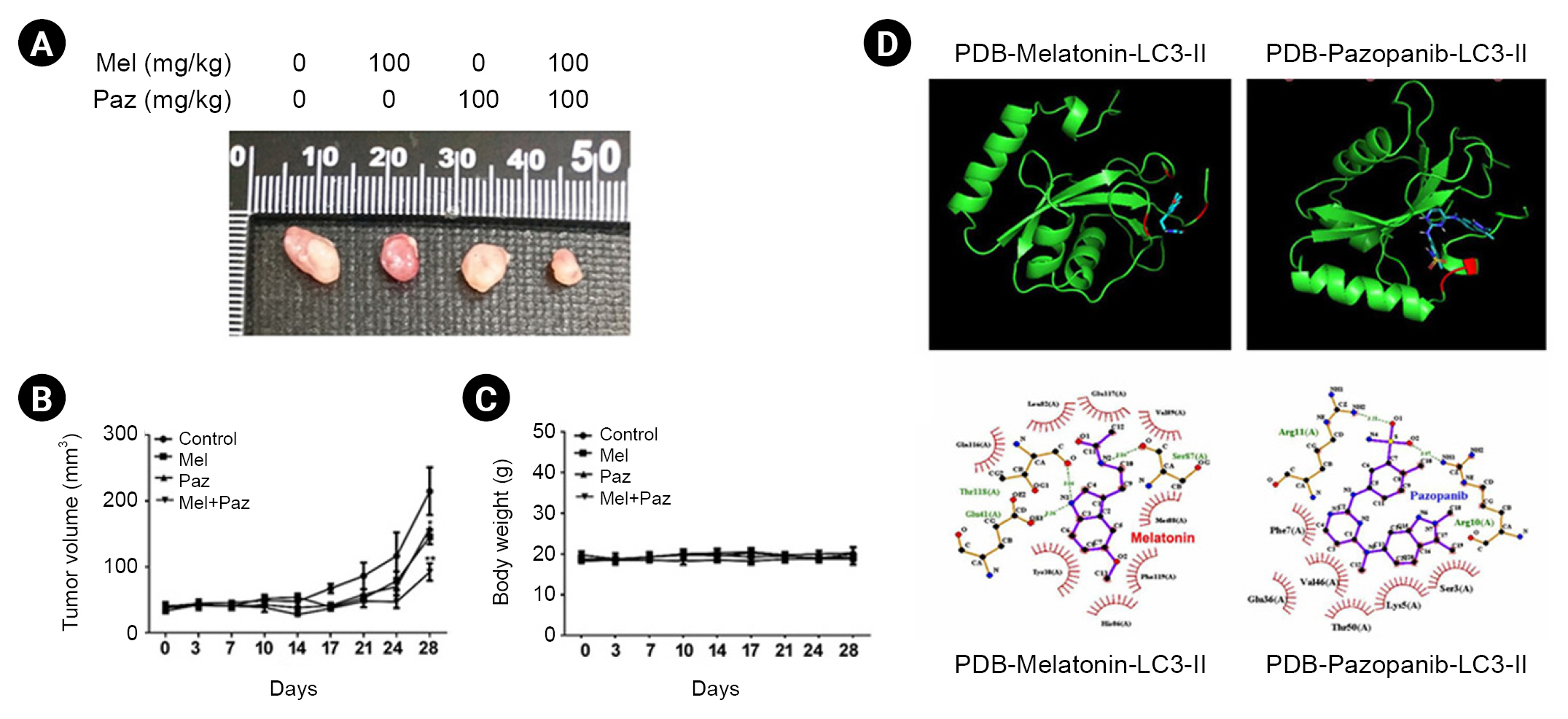
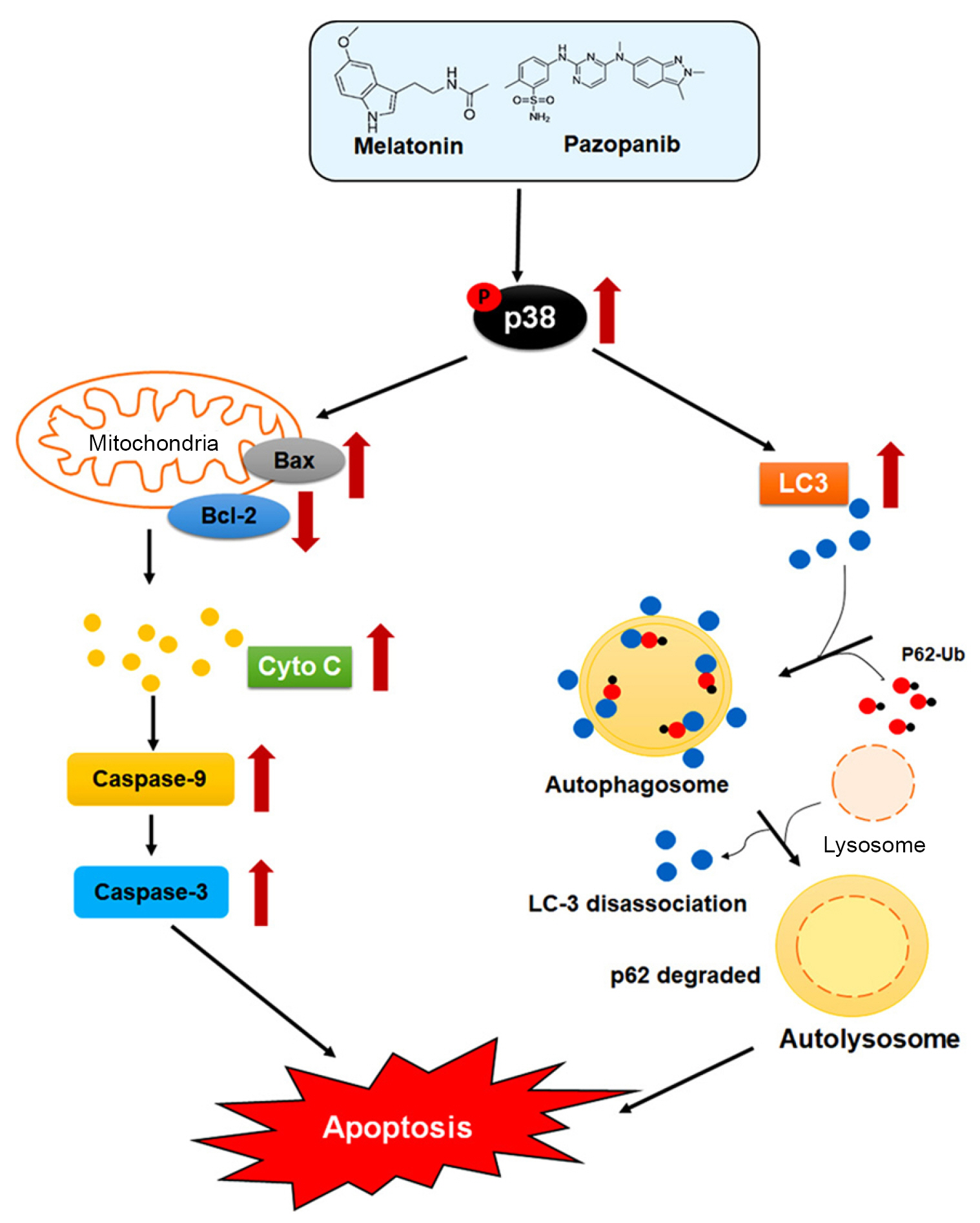
Figure 7.
Proposed model for the antitumoral activity of melatonin combined with pazopanib against human RCC cells.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Chien-Pin Lai

https://orcid.org/0000-0002-5291-0108Yong-Syuan Chen

https://orcid.org/0000-0001-5046-2594Tsung-Ho Ying

https://orcid.org/0000-0001-8244-7524Cheng-Yen Kao

https://orcid.org/0000-0003-1431-2956Hui-Ling Chiou

https://orcid.org/0000-0001-5495-2224Shao-Hsuan Kao

https://orcid.org/0000-0002-4929-3858Yi-Hsien Hsieh

https://orcid.org/0000-0003-4942-1888 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print















