Introduction
Transurethral resection of the prostate (TURP) has become a gold standard for invasive endoscopic treatment of bladder outlet obstruction [
1], i.e., benign prostatic hyperplasia (BPH) and prostate cancer. Although the procedure is relatively easy to perform, it entails a considerable risk of perioperative complications that may be acute (such as bleeding, bladder wall rupture or perforation) or chronic (such as incontinence and vesicoureteral and renal reflux) [
2].
Dilutional (hypervolemic) hyponatremia remains one of the most prevalent acute complications of TURP. In rare but life-threatening situations it may lead to TURP hyponatremia syndrome [
3]. The pathogenesis of post-TURP decreases in serum sodium has been well described, but routine risk assessment and prophylactic measures specific to the condition are still insufficient. The syndrome is caused by diffusion of hypoosmotic irrigating fluid [
3], most often tap water, into the vascular bed that leads to hemodilution, and thus a decrease in sodium concentration in the extracellular fluid. The development of hyponatremia induces homeostatic compensatory mechanisms including the inhibition of vasopressin excretion, which reduces reabsorption of water from the renal tubule, thereby increasing free water clearance.
The symptoms of TURP-related serum sodium decrease include nausea, confusion, bradycardia, increased blood pressure, visual disturbances, and, in severe cases, convulsions, pulmonary and brain edema, intussusception, and potentially death [
4]. Most of the acute symptoms are the consequence of increased brain cell volume. In order to minimize the risk of TURP-related hyponatremia, the duration of surgery should be minimized, but other factors such as volume of instilled fluid and its composition must also be controlled [
5]. However, such measures may not allow removal of the intended gland volume.
Several hormones might be risk markers for sodium disturbance after TURP, including atrial natriuretic peptide, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), and antidiuretic hormone (AVP). The assessment of AVP concentration in serum is, however, impeded by its short half-life period of only 5 to 20 minutes, and
ex vivo and
in vivo [
6] instability, together with strong binding of the hormone to blood cells. These factors prevent the measurement of serum AVP concentration in routine diagnostics [
6–
8].
Copeptin (CPP) is a 39-amino acid peptide [
9] produced and secreted with vasopressin in equimolar amounts and has good stability in circulation. Owing to these features, CPP is considered the best surrogate marker of vasopressin secretion in a range of human diseases related to its deficiency or access. CPP has been established as a reliable marker of inadequate response to hyponatremia treatment in patients with Schwartz-Bartter syndrome [
10]. Another study investigated whether the determination of serum CPP together with brain natriuretic peptide (BNP) and prohormone of BNP in patients suffering from chronic heart failure complicated by hypervolemic hyponatremia could be feasible. An increase in serum CPP concentration was linked to increased mortality, regardless of clinical symptoms of the disease. CPP proved superior to BNP and NT-proBNP in this regard, although all these markers seemed to be closely associated [
11]. Serum CPP may also predict survival in patients suffering from acute coronary syndrome, a condition that has been linked to increased vasopressin secretion [
12,
13]. In addition, assessment of CPP concentration in serum may predict the risk of outpatient mortality, irrespective of sodium concentration in plasma and dose of loop diuretics [
14].
To the best of our knowledge, there have been no studies that investigated the potential utility of serum CPP as a marker of acute sodium disturbance following surgeries with a high risk of hyponatremia, including TURP. The aim of this study was to assess the role of serum CPP as a marker of the risk of hyponatremia in patients undergoing TURP.
Methods
The study protocol had been approved by the Ethics Committee of the Medical University of Lodz and written informed consent was obtained from each individual (No. RNN/90/13/KE from April 13, 2013). The study was performed in accordance with the Declaration of Helsinki for human studies. This study is registered at ClinicalTrials.gov (NCT03912766 11/Apr/2019).
Forty-nine patients were initially enrolled. Six enrolled patients were excluded from the final per-protocol analysis due to a lack of laboratory measurements after surgery. All patients underwent a TURP at a single regional urology center. The inclusion criteria were as follows: male sex, age of >45 years, glomerular filtration rate estimated from serum creatinine with the Chronic Kidney Disease Epidemiology Collaboration formula (eGFR) of >45 mL/min, diagnosis of BPH, and lower urinary tract symptoms. Exclusion criteria included acute infection, heart failure (New York Heart Association stage 3 or 4), diabetes insipidus, nephrogenic diabetes insipidus and other sodium homeostasis abnormalities, and/or impaired consciousness, psychogenic polydipsia or alcohol abuse. Patients receiving thiazide or loop diuretics, vasopressin or its analogues, steroids, and neuroleptics any time within 7 days before surgery were also excluded.
Surgery was performed under spinal anesthesia. The irrigation fluid container was placed 60 cm above the pubic symphysis. In order to minimize prostate volume estimation error, its size was estimated with ultrasound in three dimensions by the same technician using the same Samsung X8 (Samsung Medison) equipment in all patients. The duration of surgery was defined from the insertion of a resectoscope into the urinary bladder to the removal of endoscopic instruments from the urethra. The mass of prostate gland specimens was assessed immediately after excision in order to determine the total gland volume removed. For the purpose of the study, sodium and potassium concentration was determined directly before the start and 12 hours after surgery. Serum CPP concentration was determined before and 12 hours after the end of surgery. Serum NT-proBNP concentration was determined immediately before surgery. The aliquots were centrifuged in a refrigerated centrifuge and immediately frozen at –70 °C. Other lab tests included complete blood count, serum creatinine, and urinalysis with the determination of urine specific gravity and proteinuria.
Systolic and diastolic blood pressure and heart rate were measured 2 hours before and during the surgery 15, 30, and 60 minutes after its commencement, depending on the duration of the procedure, and then 30 minutes after its completion. The amount of fluids instilled intravenously was carefully measured during TURP. The fluids infused included 0.9% NaCl which contains 153 mmoL/L sodium and Sterofundin (B. Braun) containing 140 mmol/L sodium. After surgery, each patient received 1,000 mL of 0.9% NaCl intravenously for 90 minutes. The volume of tap water used for flushing the bladder during surgery was assessed with an electronic flowmeter. The chemical composition of the tap water used during surgery was analyzed and it contained 0.28 mmol/L of sodium, 0.036 mmol/L of potassium, 24.6 mmol/L of calcium, and 0.074 mmol/L of magnesium. Serum and urine parameters were measured in the hospital laboratory with routine automated methods. Serum NT-proBNP was determined with electrochemiluminescence (ECLIA Roche Diagnostics) and serum CPP with the immunoenzymatic method (ELISA Cloud-Clone Corp.). The blood pressure before and after surgery was measured with a calibrated automatic oscillometric device (Omron M6; Omron). During surgery blood pressure was monitored with the monitor used for anesthesia.
The data are presented as an arithmetic mean ± standard deviation for normally distributed or median with interquartile range for non-normally distributed variables. The statistical significance of the within-group comparisons was assessed by paired t-test. The p-value of <0.05 was taken as significant. Correlations between the variables were calculated with the Pearson or Spearman parametric correlation coefficient depending on variable distribution. A multiple regression model was built with serum sodium change after TURP as a dependent variable. A receiver operator characteristic (ROC) curve was drawn using de Long’s method in order to determine the utility of biochemical parameters for serum sodium decrease risk estimation. The cut-off value ensuring the best sensitivity and specificity for each analyzed parameter was calculated using the Youden index. The statistical analysis was carried out with Statistica (version 13.1; TIBCO Software).
Sample size calculation was performed by using MedCalc (MedCalc Software). Power calculation showed that with a total number of 38 participants in two equal-sized subgroups, the analysis would have 80% power with significance of 0.05 for the comparison of the area under a ROC curve (AUC) with a null hypothesis value of 0.5 and expected AUC of 0.75.
Results
The baseline clinical and biochemical characteristics of the per-protocol study population are presented in
Table 1.
Table 2 shows the values of the parameters assessed at baseline and after surgery, as well as their absolute changes throughout the surgery and for 12 hours after TURP. The serum sodium concentration 12 hours after surgery was significantly lower than at baseline (p = 0.02). Although the absolute mean change in serum sodium levels during and after surgery was small, it was observed in 36 of 43 patients. Serum sodium decreased 12 hours after TURP to less than 130 mmol/L in only four patients. These patients (86, 85, 77, and 78 years old) were older than most of the study population (mean age of the whole group, 72.4 ± 9 years), but otherwise their clinical characteristics were similar to those who did not develop hyponatremia. These groups also did not differ significantly with respect to serum CPP and NT-proBNP. None of these patients had clinical signs of TURP syndrome. Serum CPP did not significantly increase from baseline to 12 hours after surgery (p = 0.34). Systolic blood pressure before surgery was significantly higher than after TURP (p < 0.001, respectively). Diastolic pressure before surgery was significantly higher (p = 0.03) than during the first 30 minutes of surgery and after surgery. Serum sodium before surgery correlated negatively with change in serum sodium for 12 hours from the start of surgery (r = –0.69, p < 0.001).
Serum CPP before surgery was negatively correlated with change in serum sodium for 12 hours from the start of surgery (R = –0.43, p = 0.004). No significant correlation was seen, however, between serum NT-proBNP concentration before surgery and change in serum sodium during and after surgery (p = 0.40).
A multiple regression model was built in order to analyze the effects of the variables that correlated linearly with the dependent variable on the variability of serum sodium concentration after surgery. Serum CPP before surgery and the duration of TURP explained most of the sodium concentration variation for 12 hours from the start of surgery (
Table 3).
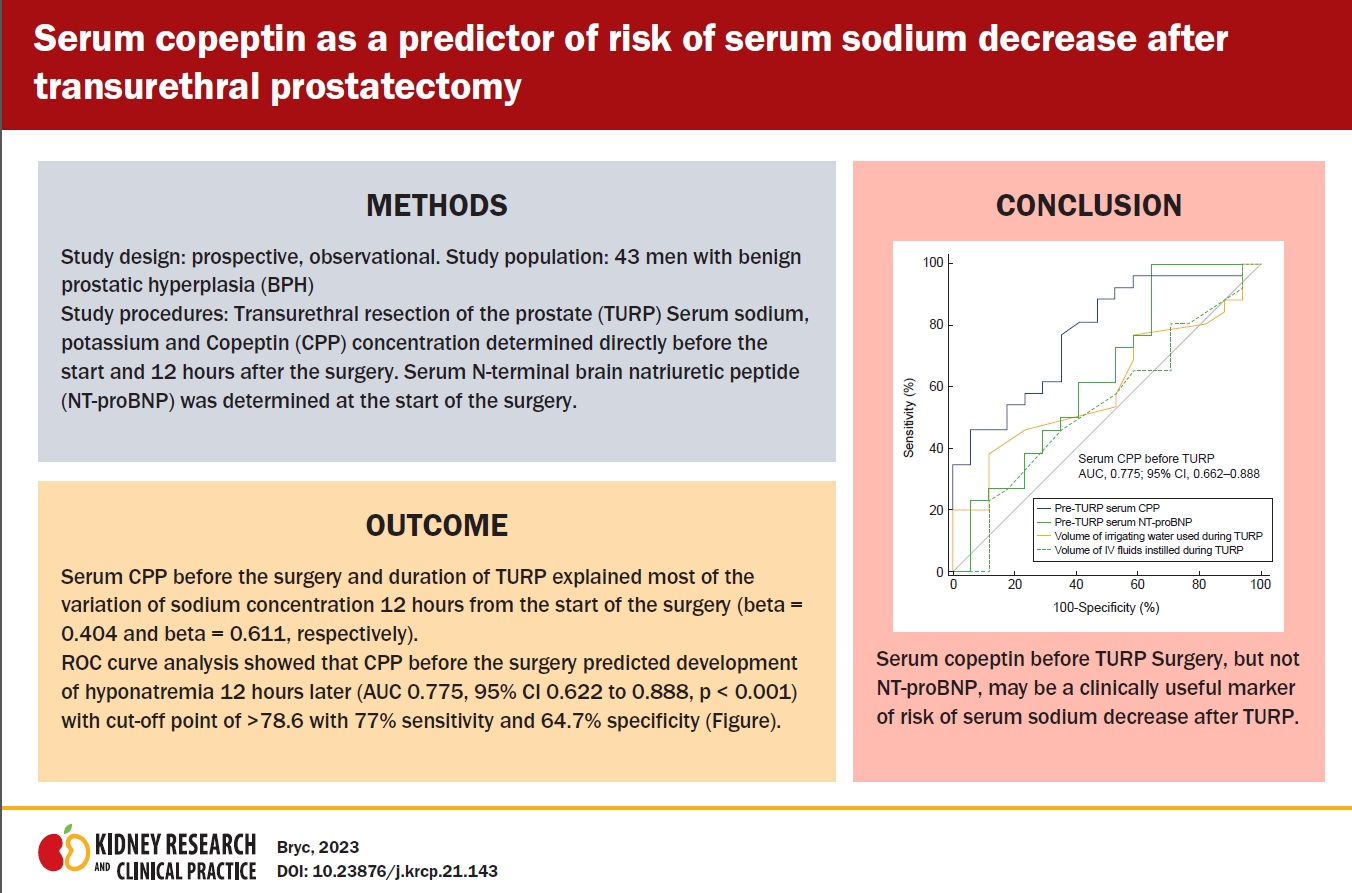
Fig. 1 shows the ROC curve with the decrease of serum sodium after surgery as a classification variable. The ROC analysis showed that serum CPP before surgery best predicted a decrease of serum sodium 12 hours after TURP (AUC, 0.775; 95% confidence interval [CI], 0.62–0.89; p < 0.001), with a cut-off point of >78.6 pg/mL, sensitivity of 77%, and specificity of 64.7%.
The plasma concentration of NT-proBNP before surgery did not predict serum sodium decrease 12 hours after TURP (AUC, 0.638; 95% CI, 0.48–0.78; p = 0.13) showing a cut-off point of ≤1,305, sensitivity of 100%, and specificity of 35.3%.
The total amount of bladder flushing fluid administered during surgery did not predict serum sodium decrease 12 hours after TURP (AUC, 0.602; 95% CI, 0.44–0.75; p = 0.24) showing a cut-off point ≤5.0, sensitivity of 38.5%, and specificity of 88.2%.
The total amount of fluid instilled intravenously during surgery predicted serum sodium decrease 12 hours after TURP with AUC of 0.0106 to 0.453 (p = 0.04). The AUC for the amount of sodium instilled intravenously was 0.543 (95% CI, 0.38–0.70), and that for serum CPP AUC was 0.775 (95% CI, 0.62–0.89).
Discussion
Despite the routine application of various measures that could prevent the development of hyponatremia during TURP such as maintaining the intrabladder pressure below 30 mmHg [
15], use of continuous-flow resectoscopes, suprapubic drainage of the bladder [
16], reduction of surgical duration to less than 1 hour, and leaving the tissue margins by the capsule of the gland where they can be left or excised completely, which reduces the duration of surgery [
17,
18], the risk of complication has not been fully eliminated and hyponatremia remains one of the most unwanted consequences of TURP surgery. Therefore, the estimation of the biochemical/hormonal markers that could allow the assessment of hyponatremia risk related to TURP remains feasible. That was why we decided to assess the utility of CPP serum concentration estimated before surgery as a potential candidate marker for calculating the development risk of postoperative hyponatremia. The results of our study confirm that the assessment of the serum concentration of CPP before surgery might be useful to predict the development of TURP-related serum sodium decrease. We have also proved that the duration of surgery correlates significantly with serum sodium decrease after TURP, which means that surgery duration is the most important determinant of hyponatremia risk. In contrast, the estimation of other potential biomarkers of hyponatremia risk, such as presurgery serum NT-proBNP concentration, was found not to be predictive in our study. That was somewhat unexpected since serum BNP and NT-proBNP concentration is a well-recognized biomarker of heart failure and overhydration [
11], and therefore we could expect that its measurement before surgery would improve the prediction of serum sodium decrease risk after TURP.
The study was comprised of a homogeneous group since we excluded the patients suffering from several chronic or acute diseases frequently leading to sodium disturbances and those taking medications that could influence the regulation of sodium balance. None of the patients suffered from infection which was particularly important since CPP is also a well-recognized marker of inflammation, including bacterial urinary tract infections [
19]. The duration of surgery could not be standardized due to different anatomical conditions and ranged from 15 to 60 minutes (median, 32.7 minutes). According to the long-term practices of our and most other centers, tap water was used to irrigate the bladder in all patients. Tap water is used mostly due to its low cost and safety, which was confirmed in numerous studies [
20]. However, the sodium concentration of tap water is much lower compared to plasma and may vary depending on location. Therefore we measured the sodium in the tap water that was used for TURP in our center. The low sodium content in tap water used for our study might have increased the risk of hyponatremia. The tap water sodium content in our country is controlled and according to the local regulations cannot exceed 8.7 mmol/L [
21], whereas the sodium content in the water used for our study was only 2.81 mmol/L.
In our study, mean serum sodium 12 hours after surgery was lower than before surgery. Similar changes were seen in other studies that investigated the utility of sodium concentration variations as a marker of TURP syndrome. In one of the studies, serum sodium concentration of >7.0 mmol/L and >7% was associated with the development of acute neurologic and cardiovascular symptoms [
22]. The surgeons performing TURP also need to carefully monitor the pressure inside the bladder, which is a major factor affecting the absorption of water from irrigation fluid. Intrabladder pressure should stay between 1 and 2.5 kPa in order to minimize the risk of dilutional hyponatremia during TURP [
23]. In our study, the irrigation fluid was administered under a controlled pressure of 60 cmH
2O (5.88 kPa), with continuous drainage of water. For technical reasons, we could not monitor intrabladder pressure during surgery.
The volume of the prostate gland was measured with ultrasound with minimized inaccuracy since the measurement was always performed by the same technician.
The main limitation of our study was a small number of patients; however, the number was higher than the minimum required as estimated via power analysis. Our research should be treated as hypothesis generating; however, we tried to overcome this limitation by recruiting a homogenous group of patients undergoing TURP. Therefore, patients with cancer were excluded in accordance with the protocol. The limitation of the study was also the lack of pressure monitoring inside the bladder during surgery. Serum CPP was measured at only two time points.
In conclusion, serum CPP measured before surgery may be a marker for the risk of hyponatremia and TURP syndrome after TURP.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















