Evaluating the Safety and effectivenesS in adult KorEaN patients treated with Tolvaptan for management of autosomal domInAnt poLycystic kidney disease (ESSENTIAL): short-term outcomes during the titration period
Article information
Abstract
Background
Tolvaptan reduces height-adjusted total kidney volume (htTKV) and renal function decline in autosomal dominant polycystic kidney disease (ADPKD). This study was aimed at investigating the efficacy and safety of tolvaptan in Korean patients with ADPKD during the titration period.
Methods
This study is a multicenter, single-arm, open-label phase 4 study. We enrolled 108 patients with ADPKD (age, 19–50 years) with an estimated glomerular filtration rate (eGFR) of >30 mL/min/1.73 m2 and factors defined as indicative of rapid disease progression. After tolvaptan titration, we evaluated efficacy and side effects and assessed factors associated with the effects.
Results
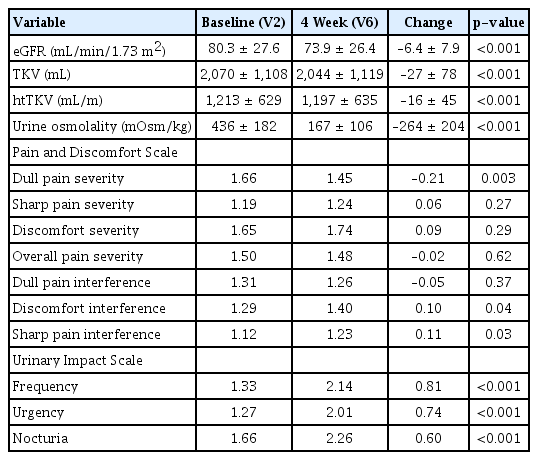
After titration for 4 weeks, eGFR and htTKV decreased by 6.4 ± 7.9 mL/min/1.73 m2 and 16 ± 45 mL/m, respectively. No serious adverse drug reactions were observed during the titration period. The greatest eGFR decline was observed in the first week, with a starting tolvaptan dose of 45 mg. Multivariate linear regression for htTKV decline showed that the greater the change in urine osmolality (Uosm), the greater the decrease in htTKV (β, 0.436; p = 0.009) in the 1D group stratified by the Mayo Clinic image classification. Higher baseline eGFR was related to a higher htTKV reduction rate in the 1E group (β, –0.642; p = 0.009).
Conclusion
We observed short-term effects and safety during the tolvaptan titration period. The decline of htTKV can be predicted as a short-term effect of tolvaptan by observing Uosm changes from baseline to end of titration in 1D and baseline eGFR in 1E groups.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the fourth most common cause of end-stage renal disease worldwide, including Korea [1,2]. The prevalence of ADPKD has been estimated to be 1 per 1,000 individuals [3,4]. ADPKD is also the most common genetic disease of the kidneys, involving the PKD1 and PKD2 genes [5]. It affects the kidney and is associated with extrarenal manifestations such as liver cysts and intracranial aneurysms [6,7]. In particular, it is characterized by large fluid-filled kidney cysts caused by increases in arginine vasopressin (AVP) level, resulting in increased intracellular adenosine cyclic monophosphate (cAMP) level in the distal tubule and collecting duct [8]. ADPKD progresses to end-stage renal disease by an average age of 60 years. Sufficient water intake is recommended to lower urine osmolality (Uosm) to 250 mOSM/kg to inhibit increases in cyst size [9]. Tolvaptan is a nonpeptide AVP V2 receptor antagonist known to induce aquaresis by decreasing the concentration of cAMP in the kidney. It has been used for water management in patients with chronic heart failure with hyponatremia and water excretion disorders, such as syndrome of inappropriate antidiuretic hormone [10–12].
Tolvaptan has been used in the management of ADPKD since the positive results of the phase 3 trial, TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3:4, were published in 2013. The TEMPO trial included 1,445 patients having ADPKD (age, 18–50 years) with a total kidney volume (TKV) of 750 mL and estimated creatinine clearance of 60 mL per minute or more. However, the proportion of Asian patients included in the TEMPO 3:4 trial was only 12.6%, and the dosage administered to Japanese participants was lower than the mean dosage for the entire population but was a higher weight-adjusted dosage [13]. Thus, there could be limitations in applying the results of the TEMPO 3:4 trial to Asian patients. Additionally, further considerations are necessary regarding aquaresis-related adverse effects and hepatotoxicity in Asian populations. Therefore, we designed this phase 4 study in a sample of Korean patients with ADPKD to determine the efficacy and safety of tolvaptan. Furthermore, we evaluated short-term effects of tolvaptan on renal function and TKV, quality of life as evaluated using the Pain and Discomfort Scale (PDS) and Urinary Impact Scale (UIS), and the incidence of adverse events during the titration period.
Methods
Ethical considerations
Informed consent was obtained from each patient at the time of enrollment. The study was approved by the institutional review board (IRB) of each participating hospital (representative hospital IRB No. H-1902-041-1009). This study was conducted according to the guidelines of the Declaration of Helsinki. The Clinical Trial registry name and registration number is ESSENTIAL trial (NCT03949894).
Study population
Patients aged 19 to 50 years who were diagnosed with ADPKD based on the unified criteria for ultrasonography-based diagnosis of the disease were enrolled [14]. Specifically, patients with an estimated glomerular filtration rate (eGFR) of >30 mL/min/1.73 m2 and rapid disease progression at the time of screening were included. Rapid progression was defined as a Mayo Clinic image classification (MCIC) of 1C, 1D, or 1E; confirmed presence of a truncating PKD1 mutation; predicting renal outcome in polycystic kidney disease (PROPKD) score greater than 6 [15]; or 2.5 mL/min/1.73 m2 or more per year for 5 years. The exclusion criteria were hyponatremia or hypernatremia, severe hepatic impairment, diabetic nephropathy or any other active glomerulonephritis, a history of hypersensitivity to benzazepine, contraindication of magnetic resonance imaging (MRI), anuria, or poor response to thirst.
Study design
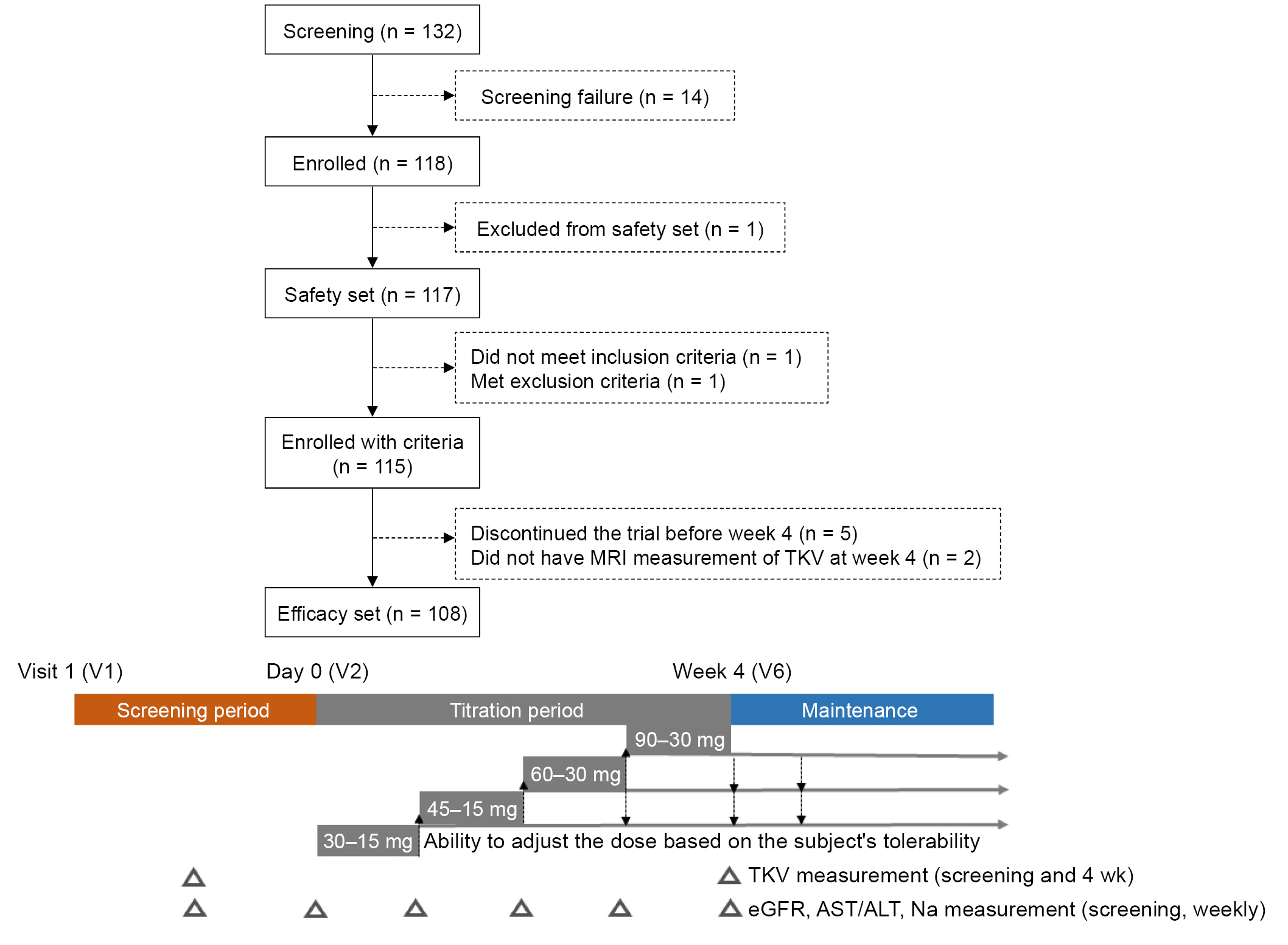
This was a multicenter, single-arm, open-label phase 4 study with screening and tolvaptan titration periods of up to 8 and 4 weeks, respectively. During the titration periods, patients visited hospitals weekly, and the dose was escalated according to the following protocol based on subject tolerability: 45 mg (30 mg + 15 mg) per day for the first week and then 60 mg (45 mg + 15 mg) per day, 90 mg (60 mg + 30 mg) per day, and 120 mg (90 mg + 30 mg) per day at intervals of at least 1 week during the tolvaptan titration period. During the maintenance period of 48 months, the patients used the highest tolerated dose among 60 mg (45 mg + 15 mg), 90 mg (60 mg + 30 mg), and 120 mg (90 mg + 30 mg) per day. Fig. 1 shows the flow chart of patient inclusion and the entire study design.
Definition of variables and outcomes
The outcomes of this study were changes in renal function, height-adjusted TKV (htTKV), patient-reported outcomes, and adverse events during the first 4 weeks of the study, which was the titration period. The evaluation was performed at the start (0 day) and the end (4 weeks) of the tolvaptan titration period. To evaluate changes in renal function, the serum creatinine level was measured at a central laboratory using the kinetic colorimetry assay, and eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. MRI was performed using a unified protocol. To minimize variability due to the multicenter trial design, each investigating institution designated one MRI scanner and team of technologists to collect trial data. The technologists completed training before the start of the trial, and a central image analysis center qualified all procedures and MRI images per a standardized MRI protocol, including sequences. MR images were acquired by the designated technologists following the MRI protocol and de-identified by each investigating institution. After receiving the deidentified MRI images, the image analysis center determined whether the images followed the MRI protocol and measured TKV centrally [16]. htTKV was defined as TKV divided by height. Uosm and creatinine were simultaneously measured at each center. Each patient underwent evaluation using the PDS and UIS during each week of the tolvaptan titration period, and the results were compared at the start (0 day) and the end (4 weeks) of the titration period to assess subjective kidney pain and urinary symptoms [17,18]. The mean prescribed tolvaptan dose was divided by body weight at each visit. Serum Na level was measured and liver function tests for aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin were measured to evaluate treatment-emergent adverse events (TEAEs). Adverse events were classified as mild, moderate, or severe. The criteria for classification were as follows: mild, discomfort without disruption to daily life; moderate, discomfort that limits or affects daily activities; and severe, inability to work or perform daily activities.
Statistical analysis
Parametric numerical variables were reported as mean ± standard deviation, and median (interquartile range) values were reported for nonparametric numerical variables. Paired t test or Wilcoxon signed-rank test was performed for comparisons before and after tolvaptan administration. The Pearson correlation coefficient was applied to determine the relationships between variables and outcomes. Multivariate linear regression analysis was used to analyze factors influencing htTKV. In the linear regression model, beta (β) was used as a standardized value to correct the units of the variables. Differences were considered statistically significant at p < 0.05.
Results
Baseline characteristics
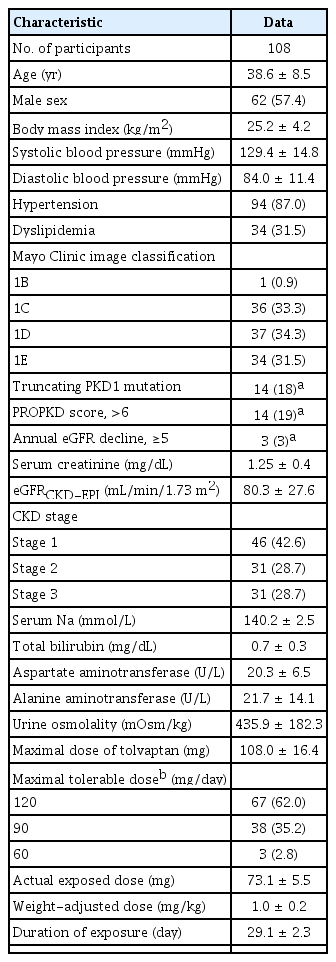
Overall, 108 patients (mean age, 38.6 ± 8.5 years) were included in the study, and 57.4% were male. The mean body mass index was 25.2 ± 4.2 kg/m2. The mean serum creatinine level and eGFRCKD-EPI were 1.15 ± 0.4 mg/dL and 80.3 ± 27.6 mL/min/1.73 m2, respectively. In total, 33.3%, 34.3%, and 31.5% of the subjects were classified as 1C, 1D, and 1E, respectively, according to the MCIC. Stage 3 chronic kidney disease (CKD) was noted in 28.7% of the patients; the average Uosm at screening was 436 ± 182 mOsm/kg. After the titration period, the mean dose was 73.13 ± 5.5 mg/day; and 62.1%, 35.1%, and 2.8% of the patients took 120 mg/day, 90 mg/day, and 60 mg/day of tolvaptan, respectively (Table 1). The titration period was temporarily discontinued for two subjects due to adverse events, and the respective doses were maintained at 60 mg and 90 mg after the titration period. In subgroups stratified by MCIC, the 1E group was younger (mean age, 32.7 ± 7.6 years; p < 0.001) and had the largest proportion of male participants (88.2%, p < 0.001). In addition, the patients in this group were taller (mean height, 1.75 ± 0.1 m; p < 0.001), heavier (mean body weight, 81.3 ± 17.3 kg; p < 0.001), and received a lower weight-adjusted dose (Supplementary Table 1, available online).
Efficacy and patient-reported outcomes during the titration period
After the 4-week titration period, eGFR significantly decreased by 6.4 ± 7.9 mL/min/1.73 m2 (p < 0.001) and htTKV by 16 ± 45 mL/m (p < 0.001) (Table 2, Fig. 2). Percentage changes in htTKV and eGFR did not differ significantly according to MCIC (Supplementary Table 1, available online). Uosm decreased by 264 ± 204 mOsm/kg after 4 weeks of tolvaptan (p < 0.001). Regarding PDS, a decrease of 0.5 points or more was defined as a meaningful decrease; however, there was no significant decrease after 4 weeks. Regarding UIS, for which the same criteria were applied, frequency (0.81 points), urgency (0.74 points), and nocturia (0.60 points) were significantly increased by tolvaptan (Table 2). During the titration period, the greatest decline of eGFR (–4.6 ± 8.4 mL/min/1.73 m2) was observed during the first week after starting tolvaptan 45 mg (Fig. 3). There was no significant difference in the amount of decrease in eGFR after the first week. This pattern was similar to that associated with CKD 1 progression to CKD 3 when analyzed according to the CKD stage (Supplementary Fig. 1B, available online). Serum Na level showed the greatest increase in the first week, with no subsequent significance. Blood pressure did not change significantly during the entire period.
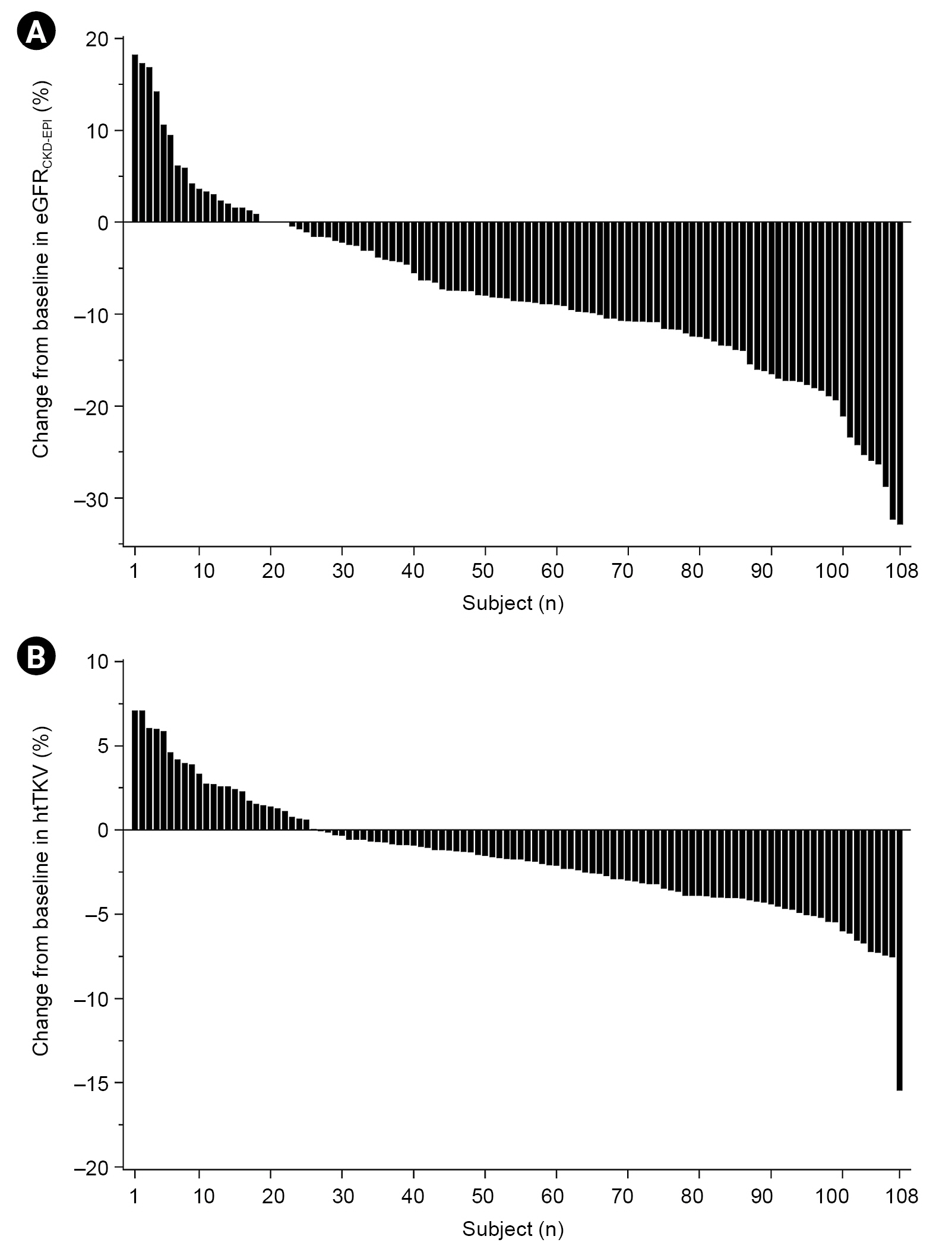
Percentage change in eGFR and htTKV from baseline.
(A) Distribution of percent change from baseline in eGFRCKD-EPI. (B) Distribution of percent change from baseline in htTKV.
eGFR, estimated glomerular filtration rate; CKD-EPI, the Chronic Kidney Disease Epidemiology Collaboration; htTKV, height-adjusted total kidney volume.

Weekly change in variables during the titration period.
(A) Mean change from baseline in eGFRCKD-EPI over time. (B) Mean serum Na over time. (C) Mean systolic blood pressure (SBP) over time. (D) Mean diastolic blood pressure (DBP) over time.
eGFR, estimated glomerular filtration rate; CKD-EPI, the Chronic Kidney Disease Epidemiology Collaboration.
Associations between variables and outcomes as tolvaptan response
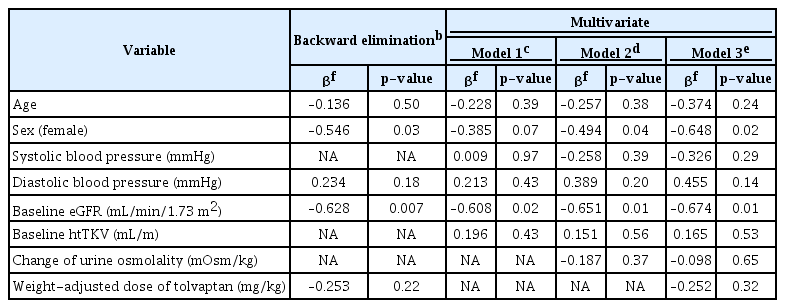
In univariate linear regression analysis, for the entire patient group, the reduction rate of htTKV was predicted by baseline Uosm (β, –0.005; p = 0.003) and change of Uosm (β, 0.004; p = 0.02) (Supplementary Table 2, available online). Additionally, baseline Uosm and change of Uosm after 4 weeks were negatively correlated in the correlation analysis (r, –0.852; p < 0.001) (Supplementary Fig. 1A, available online). In the model adjusted for age, sex, baseline htTKV, eGFR, change of Uosm, and weight-adjusted dose, significant regression was not observed. Considering the heterogeneous characteristics of patients according to MCIC (Supplementary Table 1, available online), we performed a subgroup analysis stratified by MCIC. In the subgroup analysis stratified by MCIC, the htTKV reduction rate showed a positive relationship with the decline of Uosm in class 1D (β, 0.436; p = 0.009) in the multivariable linear regression analysis adjusted for covariates such as age, sex, baseline eGFR, and baseline htTKV (Table 3, Fig. 4A). In class 1E, when all the existing variables were applied, the model did not yield significant results (F, 1.67; p = 0.20); therefore, backward elimination was performed (Table 4). Patients with preserved renal function showed a greater decrease in htTKV in class 1E (β, –0.628; p = 0.007) in the backward elimination model (F, 2.85; p = 0.03). The htTKV reduction rate after 4 weeks and baseline eGFR were negatively correlated in class 1E (Fig. 4B). Although there was a prominent decline in eGFR during the first week of the titration period, no correlations were observed in univariate linear regression for factors related to the decline of renal function during the first week (Supplementary Table 3, available online).

Correlation coefficients between variables and percentage change in htTKV.
(A) Decline in urine osmolality (Uosm) is proportional to a decrease in htTKV during the titration period in class 1D of Mayo Clinic image classification (MCIC) (n = 37). (B) Baseline eGFR is positively related to the htTKV reduction rate in class 1E of MCIC (n = 34).
htTKV, height-adjusted total kidney volume; eGFR, estimated glomerular filtration rate; CKD-EPI, the Chronic Kidney Disease Epidemiology Collaboration.
Safety and treatment-emergent adverse events during the titration period
We analyzed TEAEs in a total of 117 patients enrolled in the study. No serious adverse drug reactions were observed during the titration period. In cases of liver injury, AST and ALT levels were elevated in two patients (1.7%) and indicated mild-to-moderate severity in all patients. After maintaining the tolvaptan dose or temporarily discontinuing tolvaptan, laboratory abnormalities improved. Aquaresis, nocturia, polyuria, and urinary frequency were observed in six (5.1%), 10 (8.5%), and eight cases (6.8%), respectively, and improved in most cases (Supplementary Table 4, available online).
Discussion
This study aimed to investigate the short-term efficacy and safety of tolvaptan and to identifyc factors that can predict changes in htTKV during the tolvaptan titration period in patients with ADPKD. We found that a decrease in TKV begins during the tolvaptan titration period, and the greatest decrease in eGFR was observed during the first week of exposure to the initial tolvaptan dose (45 mg/day). Although the decrease in renal function was greatest in the first week of low-dose exposure to tolvaptan, there was no evidence of acidosis or other electrolyte abnormalities requiring discontinuation of tolvaptan through clinical assessment. This finding may be informative for clinicians considering tolvaptan prescription. We also found that the short-term effect of tolvaptan could be predicted through different factors according to MCIC. We observed that a short-term Uosm change was correlated with decline of htTKV as a response to tolvaptan. Through subgroup analysis, we found that patients classified as 1E with a higher baseline eGFR responded better to tolvaptan. In class 1E, which included younger patients and a larger proportion of male patients, initiating treatment before decline in renal function may be associated with better short-term outcomes in high-risk groups. In addition, most of the adverse events that occurred during the titration period were of mild-to-moderate severity and improved by maintaining or reducing the tolvaptan dose.
Since tolvaptan was initially found to inhibit cyst cell proliferation and cyst growth in ADPKD through in vitro and animal experiments, many studies have been conducted in humans [19,20]. Recent studies that demonstrated the long-term effects of tolvaptan are the TEMPO 3:4 and the REPRISE (Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD) trials [21,22]. As a phase 3 trial in 1,445 patients for 3 years, TEMPO 3:4 indicated that tolvaptan helped to ameliorate increase in TKV, worsening kidney function, and ADPKD-related composite events [23]. However, the proportion of the trial population made up of Asian patients was small, and there were limitations in the need for titration due to concerns about aquaresis-related adverse events and hepatotoxicity. During the titration period, it is necessary to determine the appropriate tolvaptan dose and whether there are factors that could predict the treatment response in Asians with ADPKD. Decreases in eGFR and kidney cyst size were observed in a 2011 study that reported the effects of tolvaptan administration for 1 week in 20 patients with ADPKD. While renal blood flow was preserved, the tolvaptan-induced decline in renal function was not correlated with baseline renal function [24]. Another study reported that tolvaptan was effective for increasing fractional free-water clearance even in 27 patients with decreased kidney function after 3 weeks of administration [25]. Previous studies have predicted the long-term response to tolvaptan through Uosm [26]. A greater difference in Uosm at baseline and end of titration was associated with better renal outcomes after 3 years in a post hoc analysis of the TEMPO 3:4 trial. Uosm was maintained at 200 to 300 mOsm/kg for 3 years and meant by sustained accumulating benefit. Our results also showed that Uosm change correlated with change in htTKV in the short duration of 4 weeks, suggesting its potential as a surrogate marker for predicting short-term treatment response.
Regarding the dose of tolvaptan, a daily split-dose treatment of 90 to 30 mg is currently used in consideration of pharmacokinetic results [27,28]. A previous study showed a positive relationship between weight-adjusted dose and preservation of renal function in Japanese patients [29]. An average dose of 1.14 mg/kg per day was administered, and the follow-up period was 2.52 years. The larger dose was associated with better renal outcomes. In our study, the dose of tolvaptan did not significantly affect short-term outcomes. This finding may be attributable to the difference in the weight-adjusted dose due to the difference in weight and height of the patient group according to the MCIC (1.14 ± 0.22 mg in class 1C; 1.03 ± 0.23 in class 1D; and 0.95 ± 0.21 in class 1E; p = 0.002). With progression in the MCIC, there is a possibility that the exposure dose relative to the actual body weight may decrease in this study population. In the TEMPO 3:4 trial, the protective effect on renal function appeared after 1 year, and in the ALADIN (effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease) trial, the greater the rate of decrease in renal function during the first year, the better the renal function after 3 years [30]. This was interpreted to mean that suppression of kidney compensation could be extended for better long-term outcomes. Although the observation periods differed, the pattern of decline in eGFR in the early period of tolvaptan exposure in this study was similar to the pattern of renal function change over several years in previous studies. Further studies of long-term outcomes of the ESSENTIAL trial are necessary to identify whether a decline in eGFR for 4 weeks would have a protective effect on long-term outcomes by inhibiting hyperfiltration in functional nephrons. Furthermore, we found that in 1E patients, the better the baseline renal function, the higher the htTKV reduction rate after 4 weeks. Although the difference was not significant, the preserved baseline renal function was associated with a smaller decrease in eGFR. We suggest that tolvaptan use is necessary before decrease in renal function in cases with preserved kidney function. It is unclear why the factors that predict short-term responses to tolvaptan differ according to MCIC. Depending on the stage of ADPKD, the pathophysiology of disease may be different. ADPKD is accompanied by active proliferation of cysts and inflammation in the parenchymal tissue around cysts in the early stages. As the disease progresses, interstitial fibrosis develops around cysts along with a decrease in eGFR [30]. Given these differences, change in Uosm may be a more valid surrogate marker in 1D, the early stage of the disease. In 1E, the advanced stage, initiating treatment before parenchymal fibrosis progression with eGFR decline may predict a better response.
Our study has some limitations. The first is that the protocol recommends the intake of a sufficient amount of water, but the effect of water intake could not be adjusted using plasma osmolality [31] and total Uosm could not be confirmed in the 24-hour urine test. As the time of urine sampling was variable among patients, it is possible that the pre- or post-dose regimen may have affected Uosm. In general, subjects took tolvaptan at 8 AM and 4 PM, an interval of 8 hours. Allowing modifications of that schedule depending on the subject’s living and sleep patterns may have led to intersubject variation. However, the times and intervals for taking tolvaptan were constant for each subject. Uosm was relatively constant from 4 hours to 16 hours after tolvaptan in the pharmacokinetics and pharmacodynamic study of tolvaptan [28]. The second limitation is that the change of 1.3% in htTKV is close to the range of measurement error in the ellipsoid method. Mean bias of reproducibility between observers was 0.9% in the ellipsoid method [32]. However, a decrease of 1.7% including increased htTKV in this study could be significant over 4 weeks. The third limitation is other potential confounding variables. During the study period, an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) was administered as a first-line agent in patients with systolic blood pressure of >130 and/or diastolic blood pressure of >80 mmHg to maintain the target blood pressure below 130/80 mmHg. However, medications including ACEis and ARBs could not be adjusted as covariates. Additionally, further analysis of long-term outcomes is needed to address the issue of the tolvaptan dose in ongoing phase 4 trial.
In conclusion, we observed the short-term effects and safety of tolvaptan during a 4-week titration period. We found that the greatest decrease in kidney function occurred during the first week of starting 45 mg of tolvaptan. Short-term effects of tolvaptan could be predicted using different factors according to the Mayo classification. Changes in Uosm may predict the decline of htTKV as a short-term response in 1D, while preservation of renal function before progression to parenchymal fibrosis may be related to better response in 1E, the advanced stage.
Notes
Conflict of interest
Tae-Hyun Yoo is the Editor-in-Chief of Kidney Research and Clinical Practice and was not involved in the review process of this article. All authors have no other conflicts of interest to declare.
Funding
All authors have received trial funding from Korea Otsuka Pharmaceutical Co., Ltd. All money was paid directly to the relevant institutions. The authors declare that they have no relevant financial interests.
Data sharing statement
The datasets generated and/or analyzed during the current study will be shared on reasonable request to the corresponding author.
Authors’ contributions
Conceptualization: YK, SH, YJ KYN, KBL, YKO, Hyeong Cheon Park, SHH, THY, YHK, SWK, KWL, Hayne Cho Park, SGK, HK, CHL, KTB, KHO, HJR, YCK
Data curation: YSK, WC, YK, SH, YJ, KYN, KBL, Hyeong Cheon Park, SHH, THY, YHK, SWK, KWL, Hayne Cho Park, SGK, HK, CHL, KHO, HJR, YCK
Formal analysis: HH, YSK, WC, YJ, KBL, YKO, Hyeong Cheon Park, SHH, THY, YHK, SWK, KWL, Hayne Cho Park, SGK, CHL, KTB, KHO, HJR, YCK
Funding acquisition: YKO, YCK
Investigation: HH, WC, YLK, YJ, KBL, SHH, THY, YHK, SWK, KWL, YCK
Methodology: WC, YLK, SH, Hayne Cho Park, HK
Resources: YSK, YLK, HK, CHL
Supervision: YLK, KYN, YKO, KHO, CA, YCK
Validation: YSK, KYN, YKO, SWK, Hayne Cho Park, CHL
Visualization: Hayne Cho Park
Writing – original draft: HH, HJR
Writing – review & editing: HH, KYN, YKO, KHO, CA, HJR
All authors read and approved the final manuscript.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.22.024).