| Kidney Res Clin Pract > Volume 42(2); 2023 > Article |
|
Abstract
Background
Methods
Results
Notes
Conflict of interest
Tae-Hyun Yoo is the Editor-in-Chief of Kidney Research and Clinical Practice and was not involved in the review process of this article. All authors have no other conflicts of interest to declare.
Funding
All authors have received trial funding from Korea Otsuka Pharmaceutical Co., Ltd. All money was paid directly to the relevant institutions. The authors declare that they have no relevant financial interests.
Data sharing statement
The datasets generated and/or analyzed during the current study will be shared on reasonable request to the corresponding author.
Authors’ contributions
Conceptualization: YK, SH, YJ KYN, KBL, YKO, Hyeong Cheon Park, SHH, THY, YHK, SWK, KWL, Hayne Cho Park, SGK, HK, CHL, KTB, KHO, HJR, YCK
Data curation: YSK, WC, YK, SH, YJ, KYN, KBL, Hyeong Cheon Park, SHH, THY, YHK, SWK, KWL, Hayne Cho Park, SGK, HK, CHL, KHO, HJR, YCK
Formal analysis: HH, YSK, WC, YJ, KBL, YKO, Hyeong Cheon Park, SHH, THY, YHK, SWK, KWL, Hayne Cho Park, SGK, CHL, KTB, KHO, HJR, YCK
Funding acquisition: YKO, YCK
Investigation: HH, WC, YLK, YJ, KBL, SHH, THY, YHK, SWK, KWL, YCK
Methodology: WC, YLK, SH, Hayne Cho Park, HK
Resources: YSK, YLK, HK, CHL
Supervision: YLK, KYN, YKO, KHO, CA, YCK
Validation: YSK, KYN, YKO, SWK, Hayne Cho Park, CHL
Visualization: Hayne Cho Park
Writing – original draft: HH, HJR
Writing – review & editing: HH, KYN, YKO, KHO, CA, HJR
All authors read and approved the final manuscript.
Supplementary Materials
Figure 1.
Patient inclusion flow chart and study design.
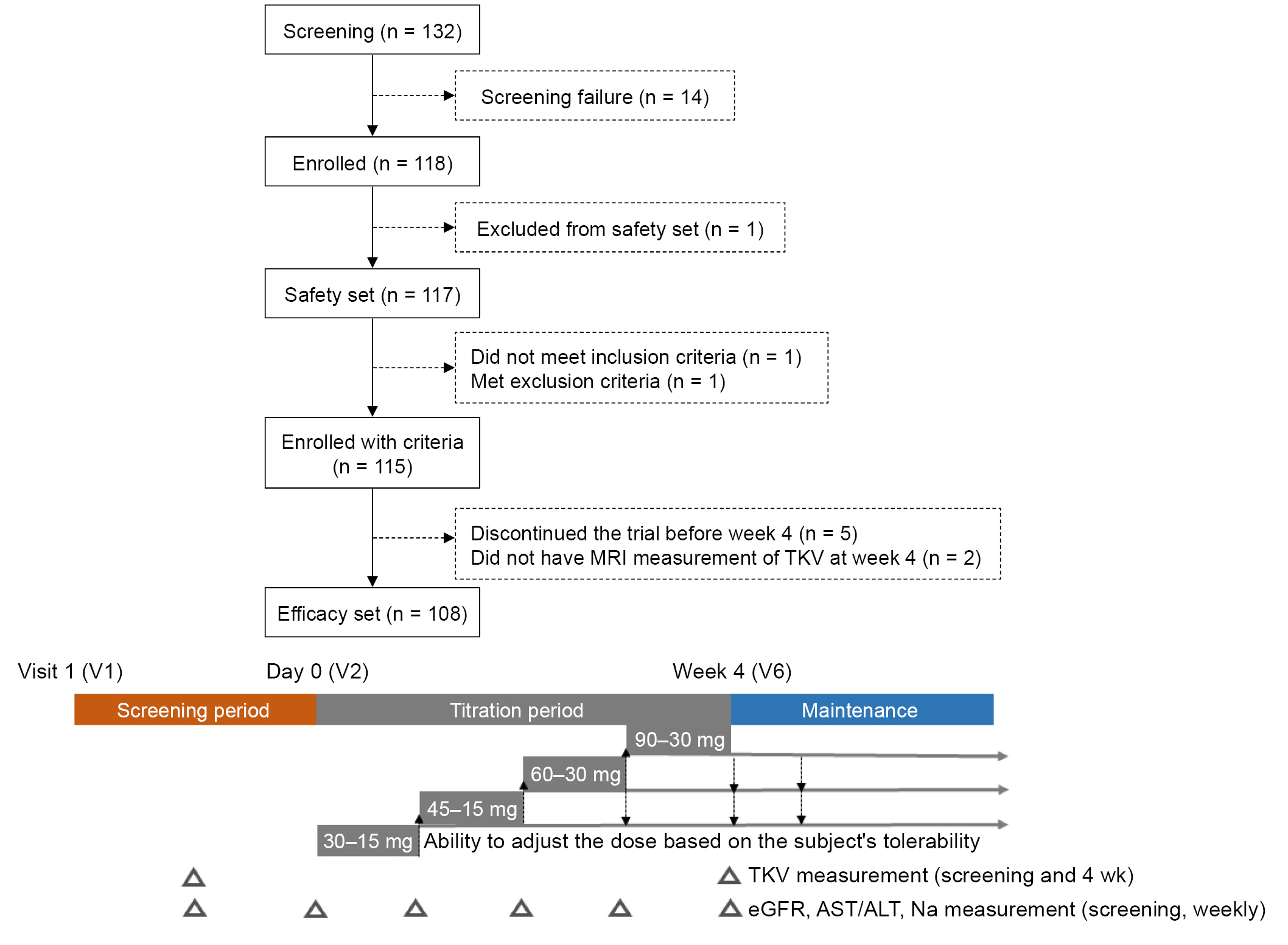
Figure 2.
Percentage change in eGFR and htTKV from baseline.
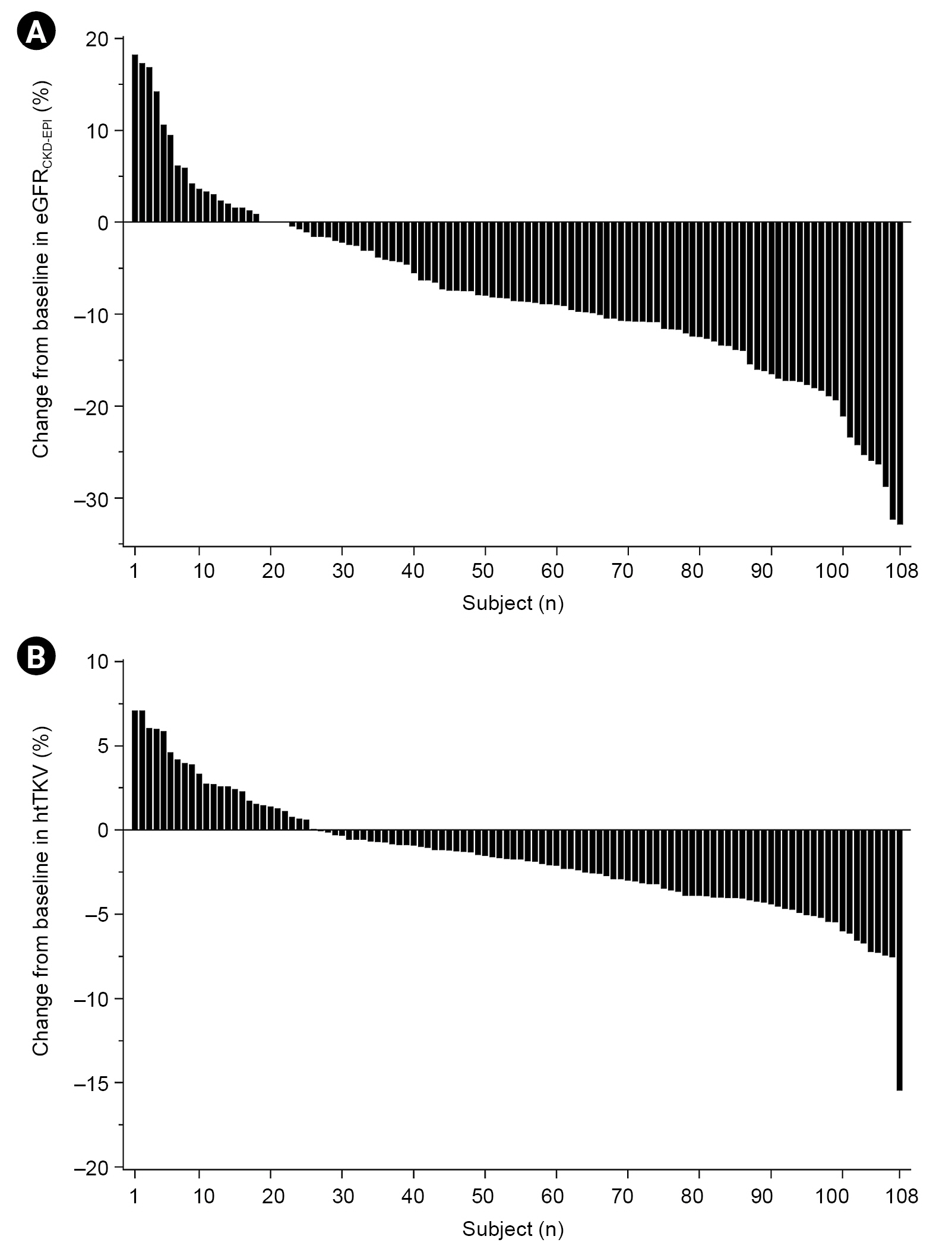
Figure 3.
Weekly change in variables during the titration period.

Figure 4.
Correlation coefficients between variables and percentage change in htTKV.

Table 1.
| Characteristic | Data |
|---|---|
| No. of participants | 108 |
| Age (yr) | 38.6 ± 8.5 |
| Male sex | 62 (57.4) |
| Body mass index (kg/m2) | 25.2 ± 4.2 |
| Systolic blood pressure (mmHg) | 129.4 ± 14.8 |
| Diastolic blood pressure (mmHg) | 84.0 ± 11.4 |
| Hypertension | 94 (87.0) |
| Dyslipidemia | 34 (31.5) |
| Mayo Clinic image classification | |
| 1B | 1 (0.9) |
| 1C | 36 (33.3) |
| 1D | 37 (34.3) |
| 1E | 34 (31.5) |
| Truncating PKD1 mutation | 14 (18)a |
| PROPKD score, >6 | 14 (19)a |
| Annual eGFR decline, ≥5 | 3 (3)a |
| Serum creatinine (mg/dL) | 1.25 ± 0.4 |
| eGFRCKD-EPI (mL/min/1.73 m2) | 80.3 ± 27.6 |
| CKD stage | |
| Stage 1 | 46 (42.6) |
| Stage 2 | 31 (28.7) |
| Stage 3 | 31 (28.7) |
| Serum Na (mmol/L) | 140.2 ± 2.5 |
| Total bilirubin (mg/dL) | 0.7 ± 0.3 |
| Aspartate aminotransferase (U/L) | 20.3 ± 6.5 |
| Alanine aminotransferase (U/L) | 21.7 ± 14.1 |
| Urine osmolality (mOsm/kg) | 435.9 ± 182.3 |
| Maximal dose of tolvaptan (mg) | 108.0 ± 16.4 |
| Maximal tolerable doseb (mg/day) | |
| 120 | 67 (62.0) |
| 90 | 38 (35.2) |
| 60 | 3 (2.8) |
| Actual exposed dose (mg) | 73.1 ± 5.5 |
| Weight-adjusted dose (mg/kg) | 1.0 ± 0.2 |
| Duration of exposure (day) | 29.1 ± 2.3 |
Table 2.
Table 3.
| Variable |
Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|---|
|
Class 1D (n = 38) |
Model 1b |
Model 2c |
Model 3d |
|||||
| β | p-value | βe | p-value | βe | p-value | βe | p-value | |
| Age | 0.050 | 0.42 | 0.127 | 0.66 | 0.359 | 0.15 | 0.359 | 0.16 |
| Female sex | –1.315 | 0.17 | –0.399 | 0.03 | –0.298 | 0.07 | –0.302 | 0.11 |
| Systolic blood pressure (mmHg) | –0.054 | 0.06 | –0.127 | 0.64 | –0.422 | 0.10 | –0.425 | 0.12 |
| Diastolic blood pressure (mmHg) | –0.066 | 0.17 | –0.434 | 0.14 | –0.155 | 0.56 | –0.15 | 0.61 |
| Baseline eGFR (mL/min/1.73 m2) | –0.008 | 0.64 | 0.059 | 0.75 | 0.11 | 0.50 | 0.107 | 0.54 |
| Baseline htTKV (mL/m) | 0.001 | 0.53 | 0.219 | 0.42 | 0.205 | 0.38 | 0.203 | 0.39 |
| Change of urine osmolality (mOsm/kg) | 0.006 | 0.02 | NA | NA | 0.435 | 0.01 | 0.436 | 0.01 |
| Weight-adjusted dose of tolvaptan (mg/kg) | 1.992 | 0.35 | NA | NA | NA | NA | –0.009 | 0.98 |
Table 4.
| Variable | Backward eliminationb |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|---|
|
Model 1c |
Model 2d |
Model 3e |
||||||
| βf | p-value | βf | p-value | βf | p-value | βf | p-value | |
| Age | –0.136 | 0.50 | –0.228 | 0.39 | –0.257 | 0.38 | –0.374 | 0.24 |
| Sex (female) | –0.546 | 0.03 | –0.385 | 0.07 | –0.494 | 0.04 | –0.648 | 0.02 |
| Systolic blood pressure (mmHg) | NA | NA | 0.009 | 0.97 | –0.258 | 0.39 | –0.326 | 0.29 |
| Diastolic blood pressure (mmHg) | 0.234 | 0.18 | 0.213 | 0.43 | 0.389 | 0.20 | 0.455 | 0.14 |
| Baseline eGFR (mL/min/1.73 m2) | –0.628 | 0.007 | –0.608 | 0.02 | –0.651 | 0.01 | –0.674 | 0.01 |
| Baseline htTKV (mL/m) | NA | NA | 0.196 | 0.43 | 0.151 | 0.56 | 0.165 | 0.53 |
| Change of urine osmolality (mOsm/kg) | NA | NA | NA | NA | –0.187 | 0.37 | –0.098 | 0.65 |
| Weight-adjusted dose of tolvaptan (mg/kg) | –0.253 | 0.22 | NA | NA | NA | NA | –0.252 | 0.32 |
References
- TOOLS
-
METRICS

- ORCID iDs
-
Hyuk Huh

https://orcid.org/0000-0001-7608-0199Yong Soo Kim

https://orcid.org/0000-0003-2152-1289Wookyung Chung

https://orcid.org/0000-0002-7693-0250Yong Lim Kim

https://orcid.org/0000-0002-1344-3455Yaerim Kim

https://orcid.org/0000-0003-1596-1528Seungyeup Han

https://orcid.org/0000-0002-7561-6534Yeonsoon Jung

https://orcid.org/0000-0003-3657-7082Ki Young Na

https://orcid.org/0000-0002-8872-8236Kyu Beck Lee

https://orcid.org/0000-0002-3904-5404Yun Kyu Oh

https://orcid.org/0000-0001-8632-5743Hyeong Cheon Park

https://orcid.org/0000-0002-1550-0812Seung Hyeok Han

https://orcid.org/0000-0001-7923-5635Tae Hyun Yoo

https://orcid.org/0000-0002-9183-4507Yeong Hoon Kim

https://orcid.org/0000-0002-4101-9993Soo Wan Kim

https://orcid.org/0000-0002-3540-9004Kang Wook Lee

https://orcid.org/0000-0003-3407-1205Hayne Cho Park

https://orcid.org/0000-0002-1128-3750Sung Gyun Kim

https://orcid.org/0000-0002-5034-0527Hyunsuk Kim

https://orcid.org/0000-0003-1889-253XChang Hwa Lee

https://orcid.org/0000-0001-9965-9304Kyongtae T. Bae

https://orcid.org/0000-0002-6304-4890Kook Hwan Oh

https://orcid.org/0000-0001-9525-2179Curie Ahn

https://orcid.org/0000-0002-7872-3319Hyun Jin Ryu

https://orcid.org/0000-0003-2148-4465Yong Chul Kim

https://orcid.org/0000-0003-3215-8681 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















