Pathological validation of the Japanese Renal Pathology Society classification and challenges in predicting renal prognosis in patients with diabetic nephropathy
Article information
Abstract
Background
Diabetic nephropathy (DN) accounts for approximately half of all cases of chronic kidney disease (CKD) and end-stage kidney disease worldwide. The Renal Pathology Society (RPS) classification has been used to predict the renal prognosis in DN. In 2018, the Japanese Renal Pathology Society (JRPS) proposed a comprehensive classification system that included pathological changes in the kidney. The clinical significance of the JRPS classification system was comparatively evaluated in the present study.
Methods
A total of 93 cases diagnosed with DN from 2009 to 2019 were enrolled. JRPS scores (J-scores) were calculated by scoring the pathological factors in the JRPS classification system and comparing them with clinical parameters.
Results
Most pathological factors constituting the J-score were significantly correlated with clinical factors. Laminated nodules were inversely correlated with estimated glomerular filtration rate. After adjusting for age, sex, body mass index, hemoglobin A1c, diabetes duration, and hypertension, CKD stage was significantly correlated with JRPS grade, nodular lesions, and exudative lesions in the multivariate logistic regression analysis. However, receiver operating characteristic curve analysis revealed that the J-score (area under the curve [AUC] = 0.639) had lower clinical significance than the traditional RPS classification system (AUC = 0.675).
Conclusion
The JRPS classification can more comprehensively reflect renal changes than the RPS classification and is correlated with renal survival. When creating a new pathological classification, arteriolar hyalinosis should not be included, whereas laminated nodules should be included.
Introduction
Diabetic nephropathy (DN), also known as diabetic kidney disease, is one of the main causes of chronic kidney disease (CKD) and end-stage kidney disease worldwide [1]. As the prevalence of diabetes increases, that of DN is also expected to increase [2]. Because the pathogenesis and progression of DN are associated with various factors, predicting the progression and prognosis of DN is challenging and has not been completely studied [3].
Albuminuria, serum creatinine, and estimated glomerular filtration rate (eGFR) are widely used as clinical indicators for predicting DN progression [4]. However, further studies are necessary to develop more comprehensive and better predictors of progression. Previous studies have investigated clinical features, novel biomarkers, and pathological changes to predict renal outcomes in DN.
The Renal Pathology Society (RPS) classification was first introduced by Tervaert et al. [5] in 2010 and has been used to predict the renal prognosis in DN. However, it mainly evaluates glomerular changes in the kidney, and many studies have suggested that tubulointerstitial changes also play a role in DN progression [6–8].
In 2018, the Japanese Renal Pathology Society (JRPS) proposed the JRPS score (J-score), which is a comprehensive DN classification system [9]. The J-score includes various pathological findings, including glomerular, tubulointerstitial, and vascular lesions. However, only a few studies have been conducted on the clinical usefulness of the JRPS classification.
We evaluated the clinical significance of the JRPS classification system and investigated whether the pathological findings observed in DN were associated with DN progression.
Methods
This study was approved by the Institutional Review Board of Yonsei University Wonju Severance Christian Hospital (No. CR320183). The requirement for informed consent was waived.
Patients
A total of 93 patients who underwent renal biopsy and were diagnosed with DN between 2009 and 2019 at Wonju Severance Christian Hospital were enrolled in the study. Periodic acid-Schiff, Jones methenamine silver, and Masson trichrome-stained slides used for diagnosing DN were reviewed by two pathologists while simultaneously reviewing other pathological factors. All cases were reclassified and scored by reviewing slides and pathological reports according to the RPS and JRPS classification systems.
Pathological factors
Pathological factors included in the RPS classification are glomerular basement membrane (GBM) thickening, mesangial expansion, nodular sclerosis, and global sclerosis. Here, the total score was calculated based on tubulointerstitial and vascular lesions, including interstitial fibrosis and tubular atrophy (IFTA), inflammatory interstitial infiltrates, arteriolar hyalinosis (AH), presence of large vessels, and arteriosclerosis. In the JRPS classification, diffuse lesions, nodular lesions, subendothelial space widening (GBM doubling), mesangiolysis, exudative lesions, polar vasculosis, global/segmental glomerulosclerosis, glomerulomegaly, IFTA, interstitial inflammation, AH, and intimal thickening are also included (Fig. 1). The J-score was calculated using the method described by Hoshino et al. [9]. Based on the J-score, the patients in the study were divided into four groups (JRPS grade 1, J-score of 0–5; JRPS grade 2, 6–10; JRPS grade 3, 11–15; and JRPS grade 4, 16–19). In addition to the pathological factors mentioned above, other pathological factors considered significant prognostic factors in DN (laminated nodules, nodular lesions with laminated appearance and capillary microaneurysms, and rupture of the capillary wall due to severe mesangiolysis) were reviewed (Fig. 2).

Microscopic findings of representative pathological prognostic factors of diabetic nephropathy.
(A) Diabetic nodules (indicated by arrows; Masson’s trichrome stain, ×400). (B) Glomerular basement membrane duplication (indicated by arrows; Jones’s methenamine silver stain, ×600). (C) Exudative lesion (indicated by arrows; periodic acid-Schiff stain [PAS], ×400). (D) Arteriolar hyalinosis (indicated by arrows; PAS, ×400).

Microscopic findings of a laminated nodule and capillary microaneurysm.
(A, B) Laminated nodule (indicated by arrows; Jones’s methenamine silver stain [JMS], ×400). (C) Capillary microaneurysm (indicated by arrows; JMS, ×400). (D) Capillary microaneurysm (indicated by arrows; periodic acid-Schiff stain, ×400).
Clinical and laboratory investigations
Data, including age, sex, body mass index (BMI), hemoglobin, serum albumin, total cholesterol, creatinine, eGFR, hemoglobin A1c (HbA1c), total urinary protein, microalbuminuria, urinary red blood cells, hypertension, diabetic retinopathy status, type of diabetes, duration of diabetes, and renal replacement therapy (RRT) status, were obtained from electronic medical records.
Statistical analysis
Descriptive statistical analyses were performed on basic patient information. For each variable, the chi-square test and independent-sample t test were performed to determine whether RRT made significant differences in outcomes. Pearson correlation coefficient analysis was performed to determine the relationships between RPS glomerular class, J-score, and JRPS grade and individual pathological factors and clinical prognostic factors. RPS glomerular classes IIa and IIb were combined as class II because of the small number of cases. The differences between mean values of the clinical factors and additional pathological factors observed in DN were analyzed using t tests. After dividing JRPS grade, RPS glomerular class, and individual pathological factor scores into two groups, the relationships between greater than 50% decrease in eGFR (eGFR 50), RRT, and CKD stage were analyzed using univariate logistic regression. Factors that showed significant relationships in the above analyses were analyzed according to other clinical elements of each patient and included in the multivariate logistic regression analysis. Finally, the clinical significances (predicting renal injury requiring RRT) of the J-score, JRPS grade, and RPS glomerular class were compared using receiver operating characteristic (ROC) curve analysis. All statistical analyses were performed using IBM SPSS for Windows, version 28.0 (IBM Corp., Armonk, NY, USA), and p-values of <0.05 were considered statistically significant.
Results
Baseline characteristics
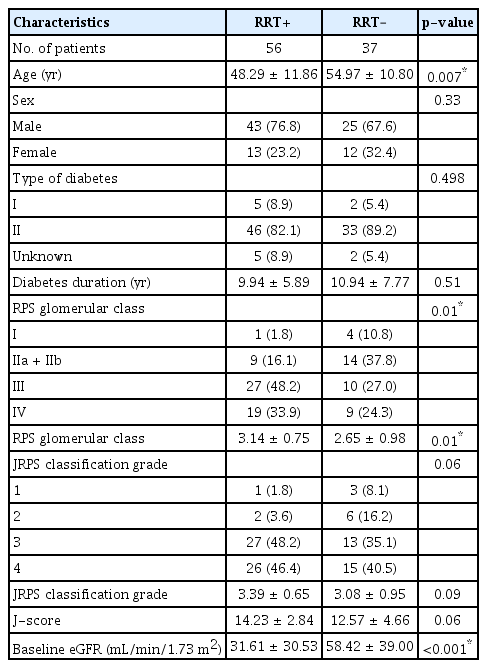
The mean age of patients was 51.00 (standard deviation [SD], ±11.85) years, and there were 25 female patients (26.9%). Additionally, seven patients (7.5%) had type I diabetes, and the mean diabetes duration was 10.33 (SD, ±6.67) years. The number of patients classified as RPS classes I, II (IIa + IIb), III, and IV were 5, 23, 37, and 28, respectively; the mean RPS class was 2.95. The number of patients classified as JRPS grades 1, 2, 3, and 4 were four, eight, 40, and 41, respectively. The mean JRPS classification grade and J-score were 3.27 (SD, ±0.80) and 13.57 (SD, ±3.74), respectively. The mean baseline eGFR was 42.28 (SD, ±36.42) mL/min/1.73 m2, and 56 patients were treated with RRT. Age and the RPS class were significantly related to whether RRT was performed (Table 1).
Correlations between pathological factors of the Japanese Renal Pathology Society classification and clinical factors
Pearson correlation coefficient analysis demonstrated that the JRPS score, comprising the sum of the scores of all pathological factors of the JRPS classification, was significantly inversely correlated with eGFR and positively correlated with CKD stage. When comparing each pathological component of the JRPS classification, GBM doubling, mesangiolysis, exudative lesion, global glomerulosclerosis, IFTA, and interstitial inflammation were positively correlated with serum creatinine level. Diffuse lesions, nodular lesions, subendothelial space widening, mesangiolysis, exudative lesions, polar vasculosis, global glomerulosclerosis, IFTA, interstitial inflammation, and intimal thickening were inversely correlated with eGFR. Additionally, diffuse lesions were positively correlated with HbA1c levels, subendothelial space widening was inversely correlated with the 24-hour urine albumin level, and nodular lesions, exudative lesions, global glomerulosclerosis, IFTA, and interstitial inflammation were positively correlated with CKD stage (Table 2).
Correlations between other pathological factors and clinical factors
When the differences between other pathological factors observed in DN and the mean values of clinical factors were compared using t tests, a statistically significant decrease in eGFR was observed when laminated nodules were present. Microaneurysms were not related to mean clinical factor values (Table 3).
Correlations between pathological factors and clinical factors
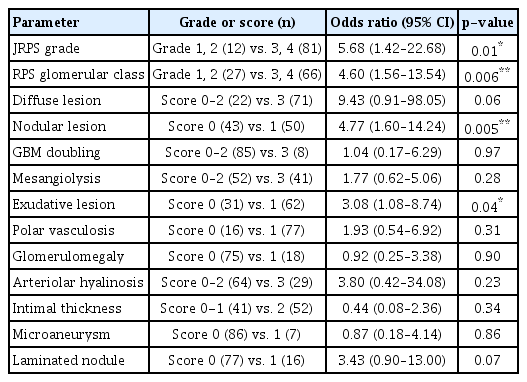
To examine relationships between the pathological prognostic factors in JRPS, RPS classification, and eGFR 50 univariate logistic regression showed that JRPS grade was significantly correlated with eGFR reduction (Table 4). Logistic regression analyses between pathological factors of the JRPS, RPS classification, and RRT demonstrated that the JRPS grade, RPS glomerular class, nodular lesions, and exudative lesions were significantly correlated with RRT (Table 5). Additionally, significant correlations were observed between CKD stage and JRPS grade, RPS glomerular class, diffuse lesions, nodular lesions, and exudative lesions (Table 6). After adjusting for age, sex, BMI, HbA1c, diabetes duration, and hypertension, CKD stage was found to be significantly correlated with JRPS grade, nodular lesions, and exudative lesions by multivariate logistic regression analysis (Table 7).
Comparisons of clinical significance between Japanese Renal Pathology Society and Renal Pathology Society classifications
ROC curve analysis revealed that the JRPS grade (area under the curve [AUC] = 0.617) had slightly lower clinical significance than the traditional RPS classification (AUC = 0.675). The overall J-score (AUC = 0.639) and J-score excluding AH (AUC = 0.644) had slightly higher clinical significance than the JRPS classification grade (Fig. 3).
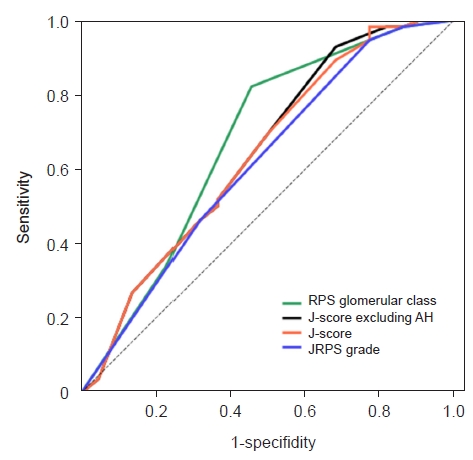
Receiver operating characteristic curve for the prediction of renal injury requiring renal replacement therapy.
The area under the curve: Renal Pathology Society (RPS) glomerular classification, 0.675; Japanese Renal Pathology Society (JRPS) grade, 0.617; JRPS score (J-score) excluding arteriolar hyalinosis (AH), 0.644; and J-score, 0.639.
Discussion
We comprehensively evaluated clinical prognostic factors and pathological findings in DN. When the clinical significance of the pathological factors included in the JRPS classification, which is a relatively recent comprehensive pathologic classification system, was analyzed, the J-score proved to be significantly correlated with CKD stage and eGFR. Most pathological prognostic factors constituting the JRPS classification were also related to clinical factors. However, the ROC curve showed that the JRPS classification had lower clinical significance than the traditional RPS classification. Among the pathological factors included in the JRPS classification, glomerulomegaly and AH were not significantly associated with clinical prognostic factors in this study. As the J-score is calculated by adding all pathological factors whether they are related or unrelated to clinical prognostic factors, the inclusion of unrelated factors may have led to the lower clinical significance of the JRPS classification. This was confirmed when the J-score was calculated without AH and found to be more significant than the overall J-score. This result suggests that the JRPS classification system requires further modification and refinement. Additional research is needed to determine the pathological findings that most accurately indicate renal damage.
Renal biopsies are performed in patients with DN when they present with atypical clinical course or when nondiabetic renal disease (NDRD) is suspected. Renal biopsy determines diagnosis and disease activity so that patients can receive appropriate treatment. Pathologists determine the correct diagnosis by comprehensively evaluating the glomeruli, tubules, interstitium, and blood vessels in the biopsy samples, as each finding provides information about the kidney and is associated with various diseases [10]. Therefore, when evaluating renal damage in DN, evaluating not only the glomeruli but also the tubules, interstitium, and blood vessels can accurately reflect renal condition. Although the JRPS classification has lower clinical significance than the RPS classification, which only evaluates the glomerulus, it more comprehensively evaluates pathological changes, similar to the ISN/RPS lupus nephritis classification and the Oxford classification for immunoglobulin A nephropathy [11,12]. Therefore, the JRPS classification considers renal damage that could be excluded by the RPS classification.
We confirmed that, except for AH, most pathological factors in the JRPS classification and J-score were related to clinical factors. However, Hoshino et al. [9] showed that AH was correlated with renal outcomes and was therefore included as a factor for calculating the J-score. Although a few studies have compared AH with individual clinical prognostic factors, Moriya et al. [13] suggested that AH is a pathological factor that can predict decreases in GFR. In a previous study by Li et al. [14], AH was not associated with renal outcomes in DN patients. As described above, the clinical significance of AH remains controversial; therefore, it may be challenging to use it as a factor for predicting the prognosis of DN. AH is not only a characteristic finding in DN but also a histological finding representative of microvascular damage caused by several factors, including hyperglycemia, hypertension, and endothelial damage, that result in glomerular damage and eventually a decrease in renal function [15,16]. Although AH is associated with decreased renal function, the present and several previous studies did not observe associations between AH and renal outcomes; therefore, more studies of the relationship between AH and clinical factors and renal outcomes are required.
Among the pathological findings observed in DN, laminated nodules showed an inverse correlation with eGFR. Meanwhile, nodular lesions in DN, also called Kimmelstiel-Wilson lesions, are observed in approximately 25% of patients with advanced DN [5] and are associated with severe renal damage, long-standing diabetes, and poor renal prognosis [17]. Some nodular lesions show lamination on methenamine silver staining; however, this is not commonly observed, and the exact incidence is unknown. In the present study, 16 out of 93 cases (17.2%) featured laminated nodules. Lamination of nodular lesions is thought to be caused by repeated mesangiolysis and mesangial damage [18,19], which indicate continuous kidney damage. Therefore, laminated nodules may help predict the prognosis of DN. Capillary microaneurysm is a rupture of the capillary walls and a pathological finding caused by mesangiolysis [20] but was not associated with clinical factors in this study.
This study has several limitations. First, this was a single-center study, and the number of patients included was small. Additionally, patients with higher RPS classifications with diabetic nodules were included, and the number of patients in each class was unevenly distributed. Although statistically significant results were obtained, further research with a larger number of cases is required. Second, the number of patients with laminated nodules was small. Laminated nodules are not a common finding, so it was difficult to include many relevant cases in this study. A detailed study of the mechanism of occurrence and the exact incidence of laminated nodules in DN is required in the future, and further research on their relationships to renal prognosis should be conducted. Third, this study was conducted without distinguishing between type 1 and 2 diabetes. The renal pathological findings in patients with type 2 diabetes are much more heterogeneous than those in patients with type 1 diabetes. NDRD is present in approximately 30% of patients with type 2 diabetes [21], and NDRD may be clinically different from DN in type 1 diabetes. If sufficient cases can be obtained, studies that examine pathological classifications should be carried out after stratification based on diabetes type. There is no existing evidence that JRPS classification is superior to RPS classification for predicting renal prognosis, and the results of this study are similar. Additionally, a disadvantage of the JRPS classification system is that it is complicated, because it evaluates comprehensive renal status, but JRPS classification system better explains current renal status. If parameters that are difficult to evaluate are adjusted, and parameters are added or deleted according to clinical significance, the JRPS classification system can more accurately evaluate the renal prognosis of DN.
In conclusion, although the pathological classifications of RPS and JRPS are tools that can predict renal progression in patients with DN, the JRPS classification can more comprehensively reflect renal changes. When developing a new pathological classification system, AH is suggested to be excluded whereas laminated nodules are suggested to be included.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2020R1F1A1049799).
Authors’ contributions
Conceptualization: TK, YK, ME
Data curation: YK, JYL, HS
Formal analysis: TK, JYL, HS, ME
Funding acquisition, Methodology, Project administration, Resources, Supervision: ME
Validation: ME, JSK, JWY
Writing–original draft: TK, JYL
Writing–review & editing: all authors
All authors read and approved the final manuscript.