| Kidney Res Clin Pract > Volume 41(Suppl 2); 2022 > Article |
|
Abstract
Notes
Figure 1.
Multidisciplinary treatment algorithm for patients with type 2 diabetes and diabetic kidney disease.
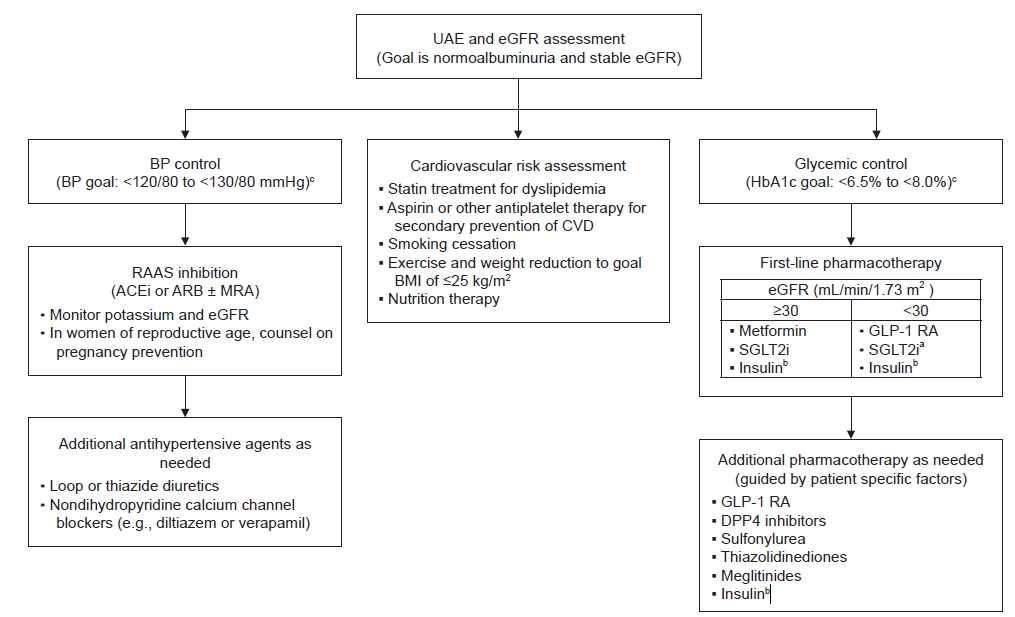
Table 1.
| Antihyperglycemic agent |
eGFR (mL/min/1.73 m2) |
|||||
|---|---|---|---|---|---|---|
| >60 | 45–60 | 30–44 | 15–29 | <15 | ||
| Biguanides | Metformin | No adjustment | No adjustment | Use with caution | Not recommended | Not recommended |
| Sulfonylureas | Glipizide | No adjustment | No adjustment | Use with caution | Use with caution | Use with caution |
| Glimepiride | Use with caution | Use with caution | Use with caution | Not recommended | Not recommended | |
| Glyburide | Use with caution | Not recommended | Not recommended | Not recommended | Not recommended | |
| Meglitinides | Nateglinide | No adjustment | No adjustment | No adjustment | No adjustment | Use with caution |
| Repaglinide | No adjustment | No adjustment | No adjustment | Use with caution | Use with caution | |
| Thiazolidinediones | Pioglitazone | No adjustment | No adjustment | No adjustment | No adjustment | No adjustment |
| Rosiglitazone | No adjustment | No adjustment | No adjustment | No adjustment | No adjustment | |
| GLP-1 receptor agonists | Dulaglutide | No adjustment | No adjustment | No adjustmenta | No adjustmenta | No adjustmenta |
| Exenatide | No adjustment | No adjustment | Use with caution | Not recommended | Not recommended | |
| Liraglutide | No adjustment | No adjustment | No adjustment | No adjustmenta | No adjustmenta | |
| Semaglutide | No adjustment | No adjustment | No adjustment | No adjustmenta | No adjustmenta | |
| Lixisenatide | No adjustment | No adjustment | No adjustment | Use with caution | Not recommended | |
| DPP4 inhibitors | Alogliptin | No adjustment | Max 12.5 mg/day | Max 12.5 mg/day | Max 6.25 mg/day | Max 6.25 mg/day |
| Linagliptin | No adjustment | No adjustment | No adjustment | No adjustment | No adjustment | |
| Saxagliptin | No adjustment | No adjustment | Max 2.5 mg/day | Max 2.5 mg/day | Max 2.5 mg/day | |
| Sitagliptin | No adjustment | No adjustment | Max 50 mg/day | Max 25 mg/day | Max 25 mg/day | |
| Vildagliptin | No adjustment | Max 50 mg/day | Max 50 mg/day | Max 50 mg/day | Max 50 mg/day | |
| SGLT2 inhibitors | Canagliflozin | No adjustment | Max 100 mg/day | Max 100 mg/day | Max 100 mg/day | Max 100 mg/day |
| Dapagliflozin | No adjustment | No adjustment | No adjustment | Use with cautionb | Use with cautionb | |
| Empagliflozin | No adjustment | No adjustment | No adjustment | Use with cautionc | Use with cautionc | |
The dose and eGFR lower bound for dosing have undergone frequent changes, especially for SGLT2 inhibitors. Please consult the most recent package insert for up-to-date information.
CKD, chronic kidney disease; DPP4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; Max, maximum recommended dose; GLP-1, glucagon-like peptide 1; SGLT2, sodium-glucose cotransporter 2; T2D, type 2 diabetes.
References
-
METRICS

- ORCID iDs
-
Li-Li Tong

https://orcid.org/0000-0001-6529-3017Sharon G. Adler

https://orcid.org/0000-0002-0247-3878 - Related articles
-
Diabetic kidney disease, revisited: where do we stand?2022 September;41(Suppl 2)
Nondiabetic kidney diseases in type 2 diabetic patients2013 September;32(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















