Korean Society of Nephrology 2022 Recommendations on controversial issues in diagnosis and management of hyponatremia
Article information
Introduction
Background and aims
Hyponatremia is the most common electrolyte disturbance in clinical practice and occurs in 15% to 30% of hospitalized patients. Severe hyponatremia prolongs hospitalization and is related to neurological prognosis and mortality due to osmotic cerebral edema. Asymptomatic-mild hyponatremia has also been reported to be associated with activities of daily living, physical and cognitive function, bone density, falls, and fractures. In addition, rapid corrective treatment of hyponatremia can cause osmotic demyelination, leading to neurological sequelae and death.
Although international guidelines for hyponatremia were published in the United States and Europe in 2013 and 2014, controversy remains due to insufficient clinical evidence. In addition, treatment can be challenging due to differences in various pathophysiological mechanisms and complex clinical settings. Since 2014 when the previous clinical practice guidelines (CPGs) were published, several studies, including randomized controlled trials (RCTs) and cohort studies, have been produced with the efforts of Korean researchers, and clinical evidence supporting treatment guidelines has been continuously added. As there is a growing need for reevaluation and updating of the related guidelines under domestic conditions, the Korean Society for Electrolyte and Blood Pressure Research (KSEBPR), in collaboration with the Korean Society of Nephrology (KSN) Clinical Practice Guideline Committee have jointly established a development committee for the process of planning, development, review, and dissemination of hyponatremia treatment guidelines in accordance with international standards. These guidelines aim to provide clinical support for shared decision-making to improve patient outcomes.
Target populations and users
This guideline applies to clinicians, patients, and subjects related to hyponatremia, and the target population includes all patients who visit medical institutions with hyponatremia. In this document, we have dealt with specific and practical contents related to diagnosis and treatment of hyponatremia. This CPG includes valuable clinical information for all medical staff, including specialists, residents, fellowships, and nurses at primary, secondary, and tertiary medical institutions managing hyponatremia in Korea. In addition, through this guideline, we also sought to provide specific and practical information to residents, fellows, nurses, and educators in leadership positions.
Methods
Organization and composition of the development committee
The KSEBPR organized the CPG development group in collaboration with the KSN, with a development working committee and a review committee consisting of nephrologists, pediatric nephrologists, and neurologists with recommendations from the KSN and its affiliated research groups (chairperson: Sejoong Kim, Seoul National University Bundang Hospital). The development working committee consisted of 18 members, including experts in the field of guideline development methodology and experienced experts in adult and pediatric nephrology who are treating patients with hyponatremia at various medical institutions. A methodology expert and a working member were included in establishing a methodology for systematic literature research and provided education on the development of clinical guidelines.
The development working group categorized the key questions into eight topics and a total of nine questions. One or two sub-chairpersons were selected for each question, and an operation meeting where all members participated was held at least once a month. Through the collaboration of members of the working-level committee, subjects to be covered in the guidelines were decided, and literature search, critical review, meta-analysis, and evidence level determination were carried out. The working committee reviewed the draft recommendation on particular topics prepared by each member. The final recommendation and its recommendation grade were determined with the consent of all members.
Patient perspective and preference
Each recommendation was reviewed by the working committee, who discussed problems that may arise in applying the recommendations to patients in the actual medical field, and who described patient values and preferences, obstacles, and facilitating factors in the text of the CPG. By presenting a plan to overcome these issues, efforts were made to balance the use of other resources with the field of diagnosis and treatment of hyponatremia in Korea.
Methodology for clinical guideline development
CPG development was carried out in four stages: planning, development, review, and dissemination. Among them, the main processes related to the development of actual recommendations can be divided into 1) selection of key questions, 2) literature search, 3) evaluation and synthesis of evidence, 4) determination of the recommendation grade and level of evidence, 5) preparation of recommendations, and 6) derivation of an agreement.
1) Review of existing international guidelines
To review domestic and international CPGs dealing with hyponatremia, we searched systematic guidelines in the last 10 years (January 2011 to December 2020) using a specific search formula (Table 1).
After reviewing the original text, the quality of the CPGs selected, including the key questions, was evaluated by two persons using the AGREE (The Appraisal of Guidelines for Research & Evaluation Instrument) II tool. The K-AGREE evaluation form developed by the Korean Medical Association was used to reduce variation among evaluators. When evaluating the quality of AGREE, to ensure the reproducibility and clarity of the evaluation result, the content that was the basis for assigning the score was written in the evaluation comment column. This underwent a revision process enabling correction (e.g., if there is a difference of 4 points or more between the reviewers). The evaluation result was derived using the scoring formula for each area. After evaluation, three treatment guidelines with a “development rigor” of >50 points among the scores for each area were selected as the CPGs for the recommendation to establish evidence.
2) Selection of key questions
The key questions were chosen by reviewing the existing CPGs from the United States and Europe, selecting detailed topics and clinical problems, reviewing the evidence for each topic, and selecting the final eight detailed topics and a total of nine questions after discussion among the working committee. Previous domestic and overseas guidelines have often been based on experience- or practice-centered recommendations. In order to compensate for the limitations of evidence-based approaches, we identified key questions based on topics that had published evidence or were relevant to recent issues. Key questions were concreted considering Population, Intervention, Comparator, and Outcome (PICO) factors and were presented in PICO format. A sentence-type key question was written, and the development possibility was reviewed and finally confirmed.
3) Preparation of a recommendation comparison table and evaluation of acceptance/applicability
After reviewing the selected CPGs, a comparison table of recommendations was made for each key question, and domestic acceptance and applicability were evaluated. The contents of the discussion were reflected in the recommendations, and a comparison table of recommendations and a table of acceptance and applicability were prepared for each key question.
4) Determining the development method
This CPG is mostly based on an adaptation of existing domestic and overseas guidelines as the primary method, and the latest research results are added. In cases where recommendations were not found in the existing guidelines, a de novo method was selectively reviewed. The adaptation development method was employed using existing CPGs as the most important source of evidence, and some systematic changes were applied to suit the medical situation in Korea.
5) Search and selection of evidence
The literature search involved major domestic and foreign literature search databases, such as Ovid MEDLINE, Ovid Embase, Cochrane library, and KMbase, among others, focusing on the keywords of each key question and an additional manual search by reviewers. The search year for the latest literature after the selected CPGs was set from 1 year before the publication of the existing evidence selected for adaptation (2012) to May 2021. A search strategy was systematically constructed with the help of a methodology expert, and a final recommendation was made by performing a search using domestic and foreign databases. The search formula is described in each recommendation’s “Search strategies” section (Supplement 1).
According to the key questions, the literature selection criteria were prepared, and two persons per individual document independently performed the first selection/exclusion and the second selection/exclusion to increase objectivity. The title and abstract of the literature were reviewed for the first screening, and for the second screening, the original text of the first selected document was reviewed. The reason for exclusion was described in the case of exclusion. If there was any disagreement between reviewers during the two-stage screening process, a consensus was reached through the consensus process (Supplement 2).
6) Preparation of evidence tables
From the selected CPGs, supporting documents for the recommendations related to the key questions of this CPG were extracted and arranged in the form of a previously agreed-upon evidence table. In addition, the evidence table was completed by adding the latest literature found through additional literature search. All documents included in the evidence table were compiled in the “Summary evidence tables” in the recommendations for each key question (Supplement 3) by conducting a risk of bias assessment appropriate to each study design, creating a risk of bias graph.
7) Bias risk assessment
The risk of bias in the included studies was evaluated using various validated checklists as recommended by the Cochrane Collaboration (Supplement 4). These were the Cochrane Risk of Bias (RoB) tool 2.0 for RCTs, Risk of Bias Assessment tool for Non-randomized Studies (RoBANS), a measurement tool for assessment of multiple systematic reviews (AMSTAR) [1] for systematic reviews (SRs), and the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) [2] for diagnostic accuracy studies.
8) Synthesis of evidence
(1) Data extraction
Documents selected from the evidence table of existing CPGs and searched papers were classified by study design, and the necessity of topics was selected from the available data list to extract the relevant contents. Data extraction was performed according to a predetermined data extraction format (data values reported in forest plots, tables, etc. were reviewed and accepted), and in the case of a comparison between the two intervention methods, a data extraction format that could evaluate the comparability was considered.
(2) Data analysis and synthesis
Meta-analysis was performed when quantitative synthesis of the extracted data was possible after completing the final evidence table, including evidence from the existing CPGs and the additional searched literature, and qualitative descriptions were made if synthesis was not possible (Supplements 5 and 6).
When meta-analysis was possible, the heterogeneity of the data was evaluated, and when the heterogeneity was judged to be high, a random-effects model was applied and subgroup analysis was performed to search for the cause of heterogeneity. Publication bias was explored by applying Egger’s test and the Trim-and-Fill method when more than ten studies were included in the synthesis. Review Manager 5.4 was used as a meta-analysis statistics program.
9) Arrangement of evidence level and recommendation grade
The level of evidence was evaluated using the Grading of Recommendations Assessment Development and Evaluation (GRADE) methodology [1–9]. The importance of each result was evaluated first, and then the level of evidence for each result was determined as one of ‘high/moderate/low/very low.’ Each evidence level definition is shown in Table 2.
The recommendation grade was divided into four levels: strong recommendation, conditional recommendation, against recommendation, and inconclusive (Table 2). As factors to consider for making recommendations, the level of evidence, benefits, risks, clinical applicability resource and cost, value, and preference were considered. Key questions that could not be adapted and developed directly due to poor existing research are expressed as an expert consensus.
10) Formulating recommendations
In formulating recommendations to improve the clinical implementation of the recommendations, the working members also reviewed the feasibility and suggestions to recommendations, such as obstacles, facilitating factors, and solutions to overcome obstacles, and then draft recommendations were made through discussion. After preparing the recommendation, it was revised through a review process via E-mail and a wired meeting with experts in the relevant field. Through in-depth discussion, the content of the recommendations and the recommendation grade was adjusted. After reflecting on the members’ review and revised opinions, the working committee described and confirmed the final recommendation level. Twelve recommendations were developed in the final eight topics.
11) Independent external review
To collect external review opinions before the publication of the developed CPG, separate from the development committee, the KSN, the Korean Society of Heart Failure, the Korean Endocrine Society, the Korean Association for the Study of the Liver, and the Korean Neurological Association, an external advisory committee composed of clinical experts and methodology experts expected to be end users of the recommended practice guidelines was formed. The advisory committee did not prepare recommendations to be included in the CPG but served as an external reviewer who consulted at the consensus stage on the derived recommendations. As a method of external review, an expert questionnaire survey was conducted to investigate the degree of consent with the recommendations for each key question. The subject of the survey was an advisory committee (including one methodology expert), and a questionnaire evaluation table was used with responses within the range of 1 point (strongly disagree) to 5 points (strongly agree) to the degree of consent to the recommendation (Supplement 7). Through the convergence, feedback was obtained, and the revised opinion was reflected in the contents of the treatment guidelines.
12) Update plan for clinical practice guideline
In the future, we will continue to derive key questions, generate recommendations based on evidence, and update existing recommendations as evidence changes. The key questions of the evidence-based CPGs will be developed based on the opinions of patients, related workers, and experts in the clinical field. Since the CPGs produced in the acceptance and adaptation method are mainly based on research conducted abroad, it is necessary to develop an appropriate recommendation for key questions fitted to the domestic situation, which should be based on domestic research results. The committee will try to promote this goal to related academic societies and seek cooperation to accumulate data. Evidence for the developed recommendation will be updated by reviewing new evidence periodically every 3 to 5 years.
13) Declaration and management of conflicts of interest
All members of the Development Committee completed a conflict-of-interest disclosure of financial or nonfinancial conflicts of interest before participating and when completing the CPG. Each member’s report of conflict of interest and management of evaluation are as shown in Supplement 8. This principle was applied from the beginning to the end of development.
Recommendations
Classification and differential diagnosis of hyponatremia
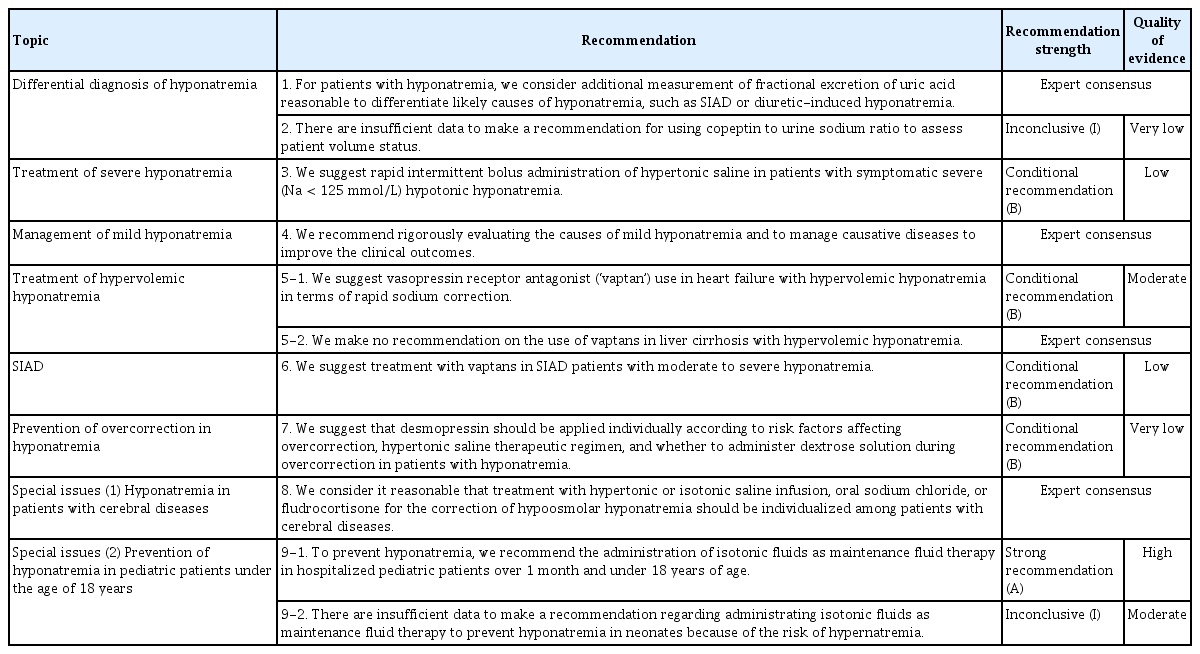
Hyponatremia is defined by less than 135 mmol/L of serum sodium (SNa) concentration [10]. Hyponatremia can be classified based on different parameters, including SNa concentration, timing of development, symptom severity, serum osmolality, and volume status. The criteria are described in Table 3 [11,12]. Because consistency and clarity of classification of hyponatremia are critical for diagnosis and management, we sought to compare the terminology used in the existing two guidelines (European and American guidelines) when discussing the classification of hyponatremia (Table 3). A practical diagnostic approach can progress step by step as follows (Fig. 1) [10].
1) Step 1
Check plasma osmolality for differentiating hypoosmolar hyponatremia from other causes of hyponatremia [10,12]. When plasma osmolality is reduced, you may require further steps of differential diagnosis. When plasma osmolality is above 275 mOsm/kg and hyponatremia is present, hyperglycemia should be checked. When serum glucose levels are increased, recheck the corrected sodium level according to the correction formula.
Corrected Na level (Hillier et al. [13]) = Na + 0.024 × (serum glucose [mg/dL] – 100)
Beyond hyperglycemia, hyperproteinemia, hyperlipidemia, and the use of mannitol or radiocontrast media can be a cause of hyper- or iso-osmolar hyponatremia [10–12].
2) Step 2
When hypoosmolar hyponatremia has been confirmed, the severity of clinical hyponatremic symptoms should be evaluated [1]. We have divided symptoms of hyponatremia into ‘asymptomatic-mild,’ ‘moderate,’ and ‘severe’ categories (Table 3). Symptomatic hyponatremia should be corrected immediately with acute management [10]. If acute management has been initiated or there are no symptoms of hyponatremia, go to the next step.
3) Step 3
Check urinary osmolality and discriminate excessive water intake.
When urinary osmolality is below 100 mOsm/kg, discriminate excessive water intake and excessive intake of hypotonic food or fluid (e.g., beer, rice wine, liquid diet) [10–12].
4) Step 4
Check urinary sodium to discriminate excessive renal excretion of sodium. When urinary sodium is above 30 mmol/L, discriminate the cause of hyponatremia according to volume status [10,12]. When volume status is decreased, check use of diuretics and cerebral salt wasting (CSW). When volume status is normal, check adrenal insufficiency, hypothyroidism, syndrome of inappropriate antidiuresis (SIAD), and other diseases or drugs that can cause SIAD.
When urinary sodium is below 30 mmol/L, recheck volume status and discriminate the causes. When volume status is decreased, check diarrhea or vomiting. When volume status is increased, discriminate congestive heart failure, liver cirrhosis, and nephrotic syndrome.
Volume status can be assessed through history-taking and physical examination. Symptoms of decreased volume status are usually nonspecific and may include thirst, fatigue, weakness, muscle cramps, and orthostatic dizziness. On physical examination, decreased skin turgor, low jugular vein pressure, orthostatic hypotension or postural tachycardia may appear. When more body fluid is lost, findings suggestive of decreased organ perfusion due to decreased intravascular fluid (low consciousness, oliguria, and peripheral cyanosis) or compensatory mechanisms (tachycardia, tachypnea, and sweating) may appear as symptoms of shock. On laboratory findings, increased urine osmolality, decreased urine sodium (UNa), alkalosis due to decreased volume status, relatively increased hemoglobin and albumin concentration may also be seen. Symptoms of increased volume status may include dyspnea on exercise, orthopnea, and peripheral edema. After underlying causes are evaluated, take further steps for managing them [10,12].
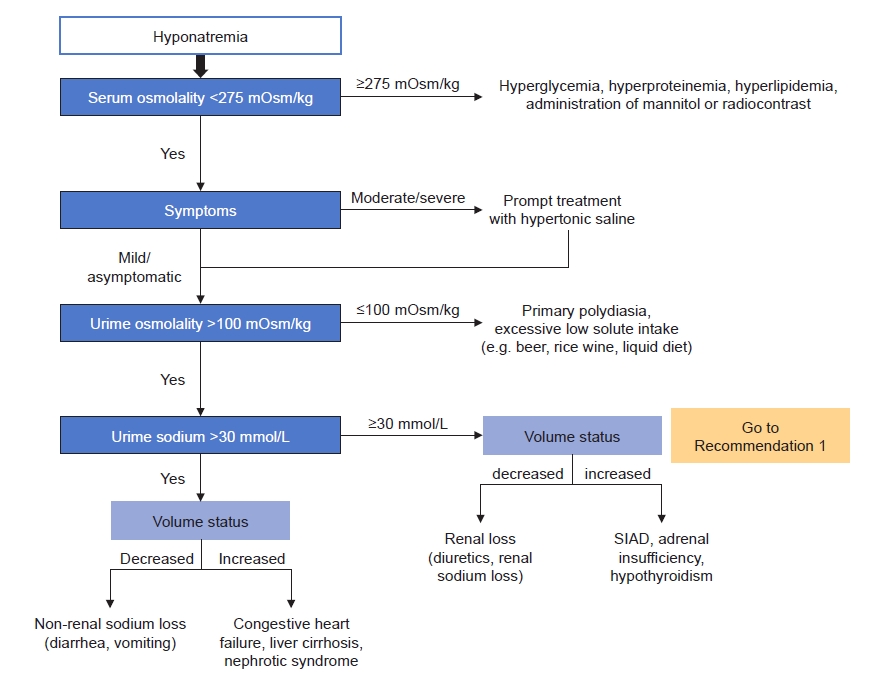
The diagnostic criteria of SIAD are summarized in Table 4 [10,11,14]. In addition, fractional excretion of uric acid (FEUA) can be used for discrimination of SIAD and use of diuretics (Recommendation 1) [12]. Serum copeptin/UNa ratio may also be used for discrimination of volume status. However, practical applications are still limited since copeptin measurement is not widely used (Recommendation 2).
Diagnostic approaches should be performed step by step, including measuring plasma osmolality, urinary osmolality, and urinary sodium levels. Patient history and physical examination are also important to discriminate underlying causes of hyponatremia. Drug history should also be checked, as it can be associated with hyponatremia including SIAD [10].
For example, thiazide diuretics are a common cause in elderly women, and desmopressin in elderly men [15,16]. In patients with chronic pain, NSAID use should be checked. In patients with skin disorders or autoimmune diseases, adrenal insufficiency should be evaluated.
Treatment of hyponatremia
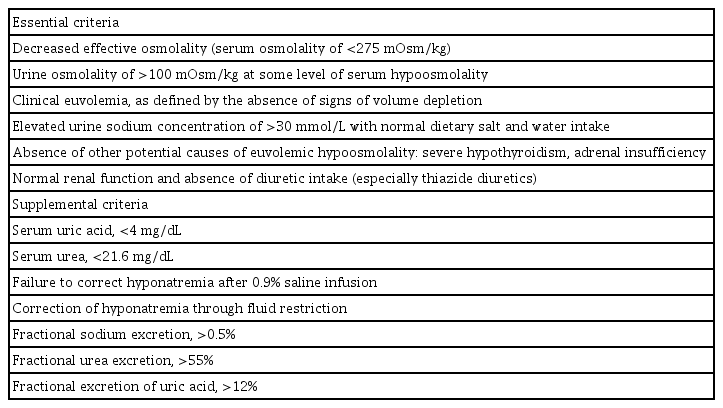
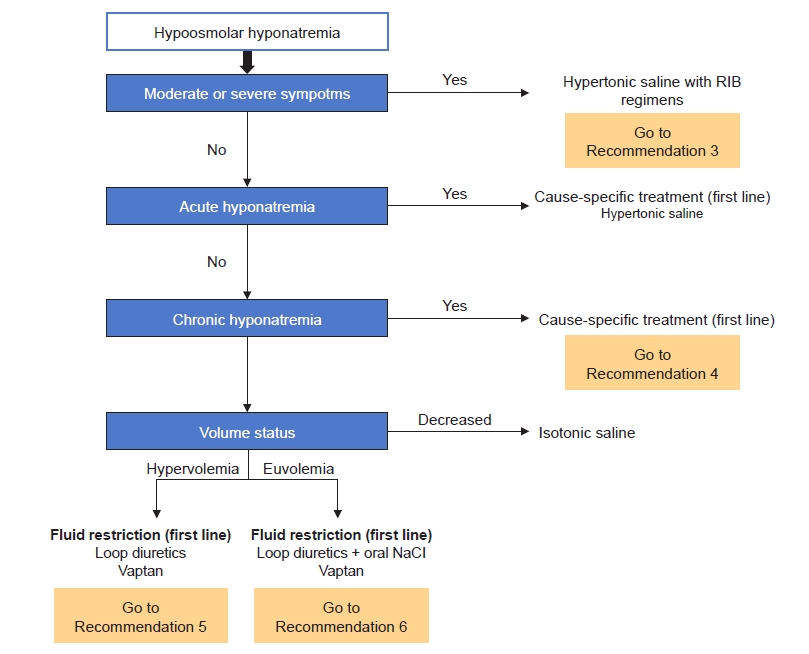
The first step treatment evaluation of hyponatremia is identifying clinical symptoms and duration of hyponatremia, as mentioned above [10]. Treatment can be approached step by step as follows (Fig. 2).
1) Symptomatic acute/chronic hyponatremia
Hypertonic saline should be administered for symptomatic hyponatremia as moderate or severe symptomatic hyponatremia reflects increased intracranial pressure. In terms of the infusion method of hypertonic saline, rapid intermittent bolus (RIB) regimens are suggested [10,11]. The treatment approach used for hypertonic saline in the American and European guidelines, and in a RCT performed in Korea is as follows (Table 5) [10–12,17]. A comparison of the efficacy and safety of hypertonic saline according to infusion methods (RIB vs. slow continuous infusion [SCI]) is discussed in Recommendation 3. In cases of severe symptomatic hyponatremia, RIB regimens of hypertonic saline should be promptly administered to increase SNa by 4 to 6 mmol/L to relieve cerebral edema, and then cause-specific treatment can be planned [10]. In cases of moderate symptomatic hyponatremia, RIB or SCI methods of hypertonic saline can be used and cause-specific treatment can be prioritized without administration of hypertonic saline [10]. We suggest checking SNa concentration 1 hour after first hypertonic saline administration, then rechecking SNa concentration every 6 hours to adjust the administration interval or infusion rate of hypertonic saline [10]. We recommend that the rate of sodium correction be reevaluated when symptoms improve or SNa concentration increases by 5 to 9 mmol/L [10,11]. If symptoms do not improve or SNa concentrations do not reach target correction, infusion of hypertonic saline may be repeated [10,11]. In patients with hypervolemic hyponatremia, hypertonic saline and loop diuretics should be administered at the same time [12].
2) Asymptomatic acute hyponatremia
The absence of moderate or severe symptoms indicates that the clinically significant brain edema has not yet developed. Therefore, prompt diagnostic assessment of hyponatremia is suggested versus immediate infusion of hypertonic saline. Nonessential fluids and medications that can contribute to or provoke hyponatremia should be stopped. If the acute decrease in SNa concentration exceeds 10 mmol/L, we suggest administering the same amount of hypertonic saline as in patients with moderate symptoms to prevent a further drop in SNa concentration [10].
3) Asymptomatic chronic hyponatremia
Asymptomatic chronic hyponatremia does not require prompt correction but may lead to localized neurologic impairment and increased mortality compared to normonatremia. Even patients with mild hyponatremia have a higher mortality rate compared to patients with normonatremia. As discussed in Recommendation 4, we found no evidence that treatment with the sole aim of correcting hyponatremia itself improves patient-relevant outcomes in mild hyponatremia. However, in this case, we should evaluate causes of hyponatremia (hypothyroidism, adrenal insufficiency, and SIAD), review medications, and recommend cause-specific treatment [10].
Hypervolemic hyponatremia is commonly seen in heart failure or liver cirrhosis. Restriction of sodium and free water intake (approximate <800–1,000 mL/day) is the first-line treatment. Additional pharmacologic therapies including loop diuretics and vasopressin receptor antagonists (‘vaptans’) can be used to increase renal free water excretion [10–12]. The possibility of using vaptans in patients with heart failure or liver cirrhosis is discussed greater detail in Recommendation 5. Fluid intake should not be restricted to prevent overcorrection when using vaptans [11].
In patients with SIAD, restricting fluid intake is the first-line treatment. The following can be considered second-line treatment: a combination of oral sodium chloride and loop diuretics or vaptans (Recommendation 6) [11,12]. NaCl causes an electrolyte diuresis by increasing urine solute load. However, its primary role is the restoration of urinary sodium losses and preventing negative sodium balance in hyponatremia [18]. NaCl is available as 1 g (17 mEq sodium and chloride) tablets. Usual doses for NaCl tablets are 6 to 9 g daily in divided doses (e.g., 2–3 g two or three times per day). Loop diuretics decrease the medullary osmotic gradient necessary for water reabsorption in the collecting duct by inhibiting the Na-K+-2Cl– cotransporter and therefore, increase free water excretion. The dose of furosemide is 20 to 40 mg per oral one time per day. They are not approved by the U.S. Food and Drug Administration (FDA) to treat hyponatremia. Daily intake of 0.25 to 0.50 g/kg urea or 600 to 1,200 mg demeclocycline can also be considered but has not been introduced in Korea.
In patients with hypovolemic hyponatremia, restoring extracellular fluid volume with intravenous isotonic fluid (0.9% saline) or balanced crystalloid will suppress vasopressin secretion causing electrolyte-free water excretion to increase [10–12]. After a 0.5- to 1.0-L infusion of isotonic fluid or balanced crystalloid, hyponatremia will begin to be corrected without signs of volume overload in patients with hypovolemic hyponatremia [11].
4) Overcorrection and re-lowering treatment of serum sodium
Target correction is achieving a SNa increase of 5 to 9 mmol/L within 24 hours and SNa of 10 to 17 mmol/L within 48 hours or reaching a SNa of 130 mmol/L within 48 hours [10]. SNa concentration should not be corrected by ≥10 mmol/L per day, with a more stringent limit of >8 mmol/L per day for patients at high risk of osmotic demyelination syndrome (ODS) (SNa concentration of ≤105 mmol/L, hypokalemia, alcoholism, malnutrition, and advanced liver disease) [11]. Overcorrection (defined as an increase in the SNa level by >12/18 mmol/L within 24/48 hours) may result in ODS [10–12]. ODS has no specific treatment and has a poor prognosis. Therefore, caution is required when correcting hyponatremia [10]. We recommend discontinuing ongoing treatment and prompt intervention to re-lower SNa concentration based on electrolyte-free water (5% glucose solutions) and/or desmopressin if overcorrection occurs (Table 5) [10–12]. Desmopressin use as a re-lowering treatment for SNa is discussed in Recommendation 7. Diuresis as a result of antagonizing vasopressin-mediated free water retention by volume repletion or discontinuing hyponatremia inducing medications often occurs when correcting hyponatremia and is a common reason for overcorrection. Therefore, urine output should be monitored during treatment.
In addition, we would like to introduce the treatment of hyponatremia in patients with brain lesions as a special situation (Recommendation 8) and selection of maintenance fluids to prevent hyponatremia in children aged ≤ 18 years (Recommendation 9) in this guideline.
Key question 1.
For patients with hyponatremia, is the additional measurement of FEUA superior to using either UNa concentration or fractional excretion of sodium (FENa) alone in differentiating SIAD?
Recommendation 1
For patients with hyponatremia, we consider additional measurement of fractional excretion of uric acid (FEUA) reasonable to differentiate likely causes of hyponatremia, such as syndrome of inappropriate antidiuresis (SIAD) or diuretic-induced hyponatremia.
Expert consensus
Remarks:
1. FEUA was significantly higher in SIAD patients than in patients taking diuretics.
2. When patients taking diuretics were divided into thiazide and loop diuretics, SIAD- and thiazide-induced hyponatremia showed similar FEUA values.
■ Rationale
FEUA is a supplemental diagnostic criterion for SIAD [14]; in patients using diuretics, FEUA performed best among UNa, FENa, fractional urea excretion, and serum uric acid concentration (area under the curve, 0.96; 0.92–1.12) [19]. In the 2013 guideline published by the American Journal of Medicine, the measurement of FEUA in patients taking diuretics has been suggested to be helpful when trying to exclude hypovolemia [11]. According to the 2014 European guideline from the European Society of Endocrinology, European Society of Intensive Care Medicine, and European Renal Association European Dialysis and Transplant Association, FEUA using a threshold of >12% was most useful for distinguishing SIAD- from non-SIAD-related hyponatremia in patients on diuretics with a sensitivity of 0.86 and specificity of 1.00 [10]. However, the previous guidelines had no evidence derived from high-quality RCTs. Our literature search identified two new observational studies from 2014 when the previous guideline was published.
In an observational study of 298 patients admitted with profound hypoosmolar hyponatremia (Na of <125 mmol/L), FEUA was higher in patients with SIAD compared with other hyponatremia etiologies (p < 0.001) [20]. We identified direct evidence from five observational studies (387 patients) that interpreted FEUA and FENa in hyponatremia patients due to SIAD and on diuretics [19–23]. Of these, one study was conducted with only patients taking thiazide diuretics, and in the other four studies, the group of patients taking thiazide or loop diuretics was not separated in our meta-analysis. A meta-analysis of studies showed that FEUA was significantly higher in SIAD patients than in patients taking diuretics. Two of five observational studies identified FEUA cutoff values of 10% and 12% (with specificity of 100% and 96%, respectively) [20,23]. Our meta-analysis found no differences in FENa. Since uric acid transporters are mostly located in the proximal tubules of the kidney, in which diuretics do not work primarily, we consider it reasonable that FEUA be used as a diagnostic test for the differential diagnosis of hyponatremia. However, caution is needed in interpreting FEUA. When patients taking diuretics were divided into thiazide and loop diuretics, SIAD- and thiazide-induced hyponatremia showed similar FEUA values in one study [23]. Furthermore, hypouricemia with increased FEUA is also observed in CSW. FEUA can be normalized after correction of hyponatremia in SIAD despite the continued increase in FEUA in CSW [24]. Lastly, concurrent use of drugs such as antihypertensives that alter uric acid excretion may affect FEUA levels [25]. Further evidence is needed to address the role of FEUA in diuretic-induced hyponatremia.
(Supplement 1| Search strategies—Key question 1.)
(Supplement 2| Study selection flow diagrams—Key question 1.)
(Supplement 3| Summary evidence tables—Key question 1.)
(Supplement 4| Bias risk assessment—Key question 1.)
(Supplement 5| Forest plots—Key question 1.)
(Supplement 6| Clinical evidence profiles [GRADE tables]—Key question 1.)
■ Recommended considerations
1) Benefits and risks
Fifteen to thirty percent of all inpatients have various degrees of hyponatremia, defined as a SNa < 135 mmol/L, which is the most frequent body fluid and electrolyte balance disturbance encountered in clinical practice [26,27]. Based on volume status, hyponatremia is classified as hypovolemic, hypervolemic, or euvolemic hyponatremia; the latter is the most common [28]. The pathophysiological cause of euvolemic hyponatremia is mainly SIAD [29]. Although most traditional diagnostic algorithms start with a clinical assessment of volume status, there are often clinical limitations in which the extracellular fluid volume status is ambiguous. In addition, the sensitivity and specificity of clinical evaluations of volume status on physical examination are low [30]. Urine osmolality and UNa concentration are prioritized over the assessment of volume status [31]. However, UNa and FENa have limited diagnostic utility in patients with complex clinical settings and varying treatment, especially on diuretics. In comparison with UNa and FENa, FEUA may be a more useful test for differentiating hyponatremia in these patients.
2) Patient values and preferences
Patients do not have a preference regarding additional measurement of FEUA. Because the choice of further laboratory measures depends mostly on medical decisions by the clinician, it is impractical to consider patient values and preferences.
3) Obstacles, facilitating factors, and measures
We expect few obstacles to accommodating these recommendations because serum and urine uric acid concentrations are not difficult to measure, and these laboratory tests are generally performed in the Republic of Korea. However, since hospitals that can provide 24-hour emergency testing for urine uric acid concentration are limited, the key is how quickly the test can be performed in clinical situations.
4) Resources
The measurement of FEUA is reimbursed under the National Health Insurance System and is already performed in most centers.
■ Other considerations
1) If the availability of urine uric acid concentration testing is limited, serum uric acid concentration may be performed for the diagnosis of SIAD [14].
2) Hypouricemia with increased FEUA is also observed in CSW. FEUA can be normalized after correction of hyponatremia in SIAD despite continued increase in FEUA in CSW [24]. Therefore, the value of FEUA as a specific tool for diagnosing SIAD is limited.
Key question 2.
In hyponatremic patients, does copeptin to UNa ratio improve differentiation of patient volume status compared with copeptin level?
Recommendation 2
There are insufficient data to make a recommendation for using copeptin to urine sodium (UNa) ratio to assess patient volume status.
Inconclusive (I), very low-quality of evidence
Remarks:
1. Copeptin levels overlap widely in hyponatremic patients and are affected by non-osmotic stimuli.
2. The ratio of copeptin to UNa was higher in disorders with secondary arginine vasopressin (AVP) release than those with primary AVP secretion such as syndrome of inappropriate antidiuresis.
■ Rationale
Assessment of volume status in hyponatremic patients is important, but often challenging. Since the sensitivity and specificity of traditional clinical assessment of patient volume status are low, there have been efforts to identify novel biomarkers. Plasma arginine vasopressin (AVP) is a promising marker for the differentiation of volume disorders from a pathophysiological perspective. However, AVP is not routinely measured in clinical practice due to its instability. Copeptin has become a surrogate maker for AVP concentration and has advantages over AVP in aspects of stability and ease of measurement. Both American and European guidelines discussed copeptin briefly [10,11]. The American (2013) and European (2014) guidelines recommend that measurement of the copeptin to UNa ratio could distinguish hypovolemic hyponatremia from SIAD and that copeptin could discriminate euvolemia from hypovolemia and hypervolemia. Both guidelines were developed based on the same observational study [32]. There were no RCTs or meta-analyses exploring the value of copeptin in patients with hyponatremia. In the present guideline, one observational study published after 2015 was added and discussed [33].
Since copeptin levels overlap widely in hyponatremic patients and are affected by non-osmotic stimuli, copeptin to UNa ratio can be useful. In a previous report of 106 German hyponatremia patients, patients were classified into five categories: 1) normal volume with excessive water intake; 2) normal volume with SIAD; 3) decreased volume due to renal sodium loss; 4) decreased or normal volume due to non-renal sodium loss; and 5) increased volume [32]. A recent study of 100 Korean hyponatremic patients also classified patients into five categories: 1) normal volume with adrenal insufficiency; 2) normal volume with SIAD; 3) decreased volume due to renal sodium loss; 4) decreased volume due to non-renal sodium loss; and 5) increased volume [33]. Both observational studies revealed that copeptin to UNa ratio was superior to copeptin level for differentiating patient volume status. The ratio of copeptin to UNa was higher in disorders with secondary AVP release (decreased effective arterial volume) than conditions with primary AVP secretion such as SIAD.
(Supplement 1| Search strategies—Key question 2.)
(Supplement 2| Study selection flow diagrams—Key question 2.)
(Supplement 3| Summary evidence tables—Key question 2.)
(Supplement 4| Bias risk assessment—Key question 2.)
(Supplement 5| Forest plots—Key question 2.)
(Supplement 6| Clinical evidence profiles [GRADE tables]—Key question 2.)
■ Recommended considerations
1) Benefits and risks
Although few observational studies evaluated the role of copeptin to UNa ratio in patients with hyponatremia, it could help differentiate the etiology of hyponatremia. Furthermore, a recent study revealed the role of copeptin as a predictor of treatment response to hypertonic saline infusion in hyponatremic patients [33].
2) Patient values and preferences
Patients do not have preferences between two diagnostic tests. The choice depends mainly on clinical decisions made by their doctor.
3) Obstacles, facilitating factors, and measures
UNa testing is available in most secondary and tertiary hospital in Korea. However, copeptin testing is only available for clinical research in Korea. Copeptin measurements has been shown to be useful as a diagnostic and prognostic biomarker in patients with lower respiratory tract infection, heart disease and cerebrovascular accident in addition to hyponatremia [34]. Further large-scale trials may facilitate the development of commercial measurement of copeptin in Korea.
4) Resources
Copeptin testing is not commercially available in Korea. To measure copeptin in Korea, specialized testing equipment (e.g., Thermo Scientific Kryptor) is required, and cost of the test is about 45,000 Korean won (KRW) per case. Cost-effectiveness is a remaining question regarding copeptin measurement in clinical practice.
Key question 3.
In patients with symptomatic severe hypotonic hyponatremia, are there any differences in symptom relief, correction of sodium, target correction rate, overcorrection, ODS, and mortality between RIB of hypertonic saline and SCI?
Recommendation 3
We suggest rapid intermittent bolus (RIB) administration of hypertonic saline in patients with symptomatic severe hypotonic hyponatremia.
Conditional recommendation (B), low-quality of evidence
Remarks:
In the treatment of symptomatic severe hypotonic hyponatremia,
1. RIB administration of hypertonic saline can effectively relieve symptoms within 12 hours compared to slow continuous infusion (SCI).
2. RIB is more effective in increasing serum sodium (SNa) within 1 hour and reaching the target correction rate than SCI.
3. RIB can result in a lower incidence of therapeutic re-lowering of SNa than SCI.
4. RIB has similar overcorrection, osmotic demyelination syndrome, and mortality rates to SCI.
■ Rationale
Hypertonic saline has been used to treat symptomatic severe hypotonic hyponatremia. Overcorrection from indiscriminate prolonged use of hypertonic saline may result in irreversible neurologic sequelae from ODS, whereas under-correction of hyponatremia may insufficiently improve fatal complications of cerebral edema. Therefore, appropriate correction of SNa is needed. Although the American (2013) and European (2014) guidelines recommend administering hypertonic saline in small, fixed boluses (recommendation grade: expert opinion in the American guidelines, 1D in the European guidelines), they were not based on high-quality RCT evidence [10,11,17]. In order to examine whether RIB therapy of hypertonic saline has any benefit for symptom relief, correction of SNa, complications, and prognosis compared to SCI in patients with symptomatic severe hypotonic hyponatremia, we reviewed a prospective cohort study (24 hours of follow-up after treatment) and a RCT (48 hours of follow-up after treatment) published after the European guideline (2014).
A prospective cohort study reported that the RIB group had more rapid elevation of SNa and greater improvement in the Glasgow Coma Scale (GCS) at 6/12 hours than the SCI group. However, there was no difference between the two groups in GCS improvement at 24 hours [35].
A RCT demonstrated that the RIB group had the higher increment in SNa at 1 hour, a higher proportion meeting the target correction rate (achieving SNa of 5–9 mmol/L within 24 hours and SNa of 10–17 mmol/L or ≥130 mmol/L within 48 hours) at 1 hour, lower SNa at 12 hours, and a lower incidence of re-lowering treatment (5% dextrose infusion 10 mL/kg over 1 hour and/or intravenous desmopressin 2 µg if SNa level increase is ≥10 mmol/L within the first 24 hours or ≥18 mmol/L within 48 hours) than the SCI group [17].
In both studies, the target correction rate, the degree of SNa elevation at 24 hours, and overcorrection (increase in SNa by >12 mmol/L within the first 24 hours or increase in SNa by >18 mmol/L within 48 hours) did not differ between the two groups [17,35]. ODS did not occur in either study [17,35]. Death occurred in five patients in the RCT and four patients in the prospective cohort study, with no significant difference between the two groups [17,35]. Only one RCT for hypertonic saline infusion in symptomatic severe hypotonic hyponatremia has been reported in Korea; thus, additional large-scale RCTs are needed.
(Supplement 1| Search strategies—Key question 3.)
(Supplement 2| Study selection flow diagrams—Key question 3.)
(Supplement 3| Summary evidence tables—Key question 3.)
(Supplement 4| Bias risk assessment—Key question 3.)
(Supplement 5| Forest plots—Key question 3.)
(Supplement 6| Clinical evidence profiles [GRADE tables]—Key question 3.)
■ Recommended considerations
1) Benefits and risks
RIB hypertonic saline treatment is a simple (user-friendly) method and has the advantage of SNa elevation within 1 hour and symptom relief within 12 hours; it also does not increase the risk of overcorrection, ODS, or death compared to SCI. The RIB group has a safety advantage, showing a reduced incidence of re-lowering treatment. There were no differences in volume overload or phlebitis caused by the infusion of hypertonic saline between RIB and SCI groups, so there is no additional risk according to the infusion method of hypertonic saline [17].
2) Patient values and preferences
From the patient’s point of view, patients do not have preferences regarding a specific method between RIB and SCI. The choice between RIB and SCI in symptomatic severe hypotonic hyponatremia depends mostly on medical decisions made by the physician. However, RIB administration might be more effective for improving the initial symptoms of hyponatremia.
3) Obstacles, facilitating factors, and measures
Education regarding the RIB method is required as domestic medical staff (doctors and nurses) are not yet familiar with RIB compared to SCI. The infusion equipment for RIB therapy is also needed. However, RIB regimens are simple and require a lower medical burden owing to the omission of the need for calculation from the physician’s perspective. They also have the advantage of shortening the careful observation time needed as the hypertonic saline infusion time is short, reducing the burden on nurses. Most domestic secondary and tertiary hospitals treating patients with severe hyponatremia already have infusion pump equipment.
4) Resources
In an RCT, the amounts of hypertonic saline required in both groups to reach an appropriate SNa concentration were similar. In a prospective cohort study, less volume of hypertonic saline was administered in the RIB group than in the SCI group. During the treatment of hyponatremia, re-lowering treatment of SNa may be needed to avoid overcorrection, which is based on infusion of electrolyte-free water and intravenous desmopressin. In an RCT, the RIB group exhibited reduced additional costs and labor because of a lower incidence of re-lowering therapy [17].
Key question 4.
For patients with mild hyponatremia (Na of 130–135 mmol/L), does the correction of SNa improve clinical outcomes, such as survival and complications, compared to untreated controls?
Recommendation 4
We recommend rigorously evaluating the causes of mild hyponatremia and to managing causative diseases to improve clinical outcomes.
Expert consensus
Remarks:
1. Mild hyponatremia increases the risk of mortality compared with those with normonatremia.
2. There is no clear evidence that correcting hyponatremia itself improves patient-important outcomes.
3. There are insufficient data to make a recommendation regarding treating mild hyponatremia with hypertonic saline or oral sodium chloride solely to increase serum sodium concentration.
■ Rationale
In the case of mild hyponatremia, it is often unnoticed in clinical practice because it rarely presents with specific symptoms. Moreover, there is lack of evidence from RCTs that treatment of mild hyponatremia with fluid therapy or medication with the sole aim of correction to normal SNa concentration improves patient outcomes. The European guideline (2014) recommends against treatment simply to increase SNa concentration (grade of recommendation 2C). However, several observational studies have shown that mild hyponatremia increases the risk of mortality both in short-term and long-term follow-up. Waikar et al. [36] reported that hospitalized patients with mild hyponatremia defined as a SNa concentration of 130 to 134 mEq/L showed increased in-hospital mortality risk compared with those with normonatremia. Doshi et al. [37] found that hospitalized cancer patients with mild hyponatremia showed two-fold increased risk of morality compared to those with normonatremia. Furthermore, Kovesdy et al. [38] observed that mild hyponatremia defined as 130 to 135.9 mEq/L increased the risk of relatively long-term mortality (median follow-up duration of 5.5 years) in patients with chronic kidney disease with estimated glomerular filtration rate less than 60 mL/min/1.73 m2. From a meta-analysis including above studies that compared the short-term (less than 90 days or in-hospital mortality) and long-term (more than 5 years mortality) mortality risk, mild hyponatremia increased both short-term (odds ratio [OR], 2.09; 95% confidence interval [CI], 1.90–2.30; p < 0.001) and long-term (OR, 1.46; 95% CI, 1.44–1.48; p < 0.001) mortality risk compared with normonatremia.
In a domestic retrospective study [39], improved SNa concentration at discharge had the strongest association with long-term mortality in acute myocardial infarction patients with hyponatremia. However, because mild hyponatremia patients were not distinguished from other study patients, and interventions such as hypertonic saline were not addressed in this study, these findings were not included in the rationale under the consensus of the Development Committee. Although there is insufficient data that the correction of mild hyponatremia with the sole aim of correcting SNa concentration has clinical benefit, it is reasonable to rigorously evaluate the causes of mild hyponatremia and to manage the diseases because mild hyponatremia increases the risk of mortality.
(Supplement 1| Search strategies—Key question 4.)
(Supplement 2| Study selection flow diagrams—Key question 4.)
(Supplement 3| Summary evidence tables—Key question 4.)
(Supplement 4| Bias risk assessment—Key question 4.)
(Supplement 5| Forest plots—Key question 4.)
(Supplement 6| Clinical evidence profiles [GRADE tables]—Key question 4.)
■ Recommended considerations
1) Benefits and risks
Since no RCTs have been published to date, correction of mild hyponatremia with hypertonic fluids is not recommended. However, as mentioned above, it is clear that mild hyponatremia is associated with poor clinical outcomes, as confirmed by previous observational studies and meta-analysis in this guideline. Therefore, we concluded that evaluation and management for causes of hyponatremia have benefits, versus treatment with the sole aim of correction of SNa concentration.
2) Patient values and preferences
Fluid therapy is usually prescribed based on the decision by the healthcare provider regarding medical necessity; thus, patient values and preferences are not a general consideration.
3) Obstacles, facilitating factors, and measures
Mild hyponatremia is often unnoticed in clinical practice due to its lack of specific symptoms. To overcome the poor prognosis associated with mild hyponatremia, we recommend to rigorously evaluating the causes of mild hyponatremia and to raise awareness of mild hyponatremia.
4) Resources
None.
Key question 5.
Do vaptans offer additional benefits compared with loop diuretics in terms of mortality, renal function, and SNa concentration in hypervolemic hyponatremia?
Recommendation 5
1. We suggest vaptan use in heart failure with hypervolemic hyponatremia in terms of rapid sodium correction.
Conditional recommendation (B), moderate-quality evidence
2. We make no recommendation on the use of vaptans in liver cirrhosis with hypervolemic hyponatremia.
Expert consensus
Remarks:
1. We evaluated the efficacy of adding vaptans to loop diuretics since few studies compared vaptans versus loop diuretics in heart failure with hypervolemic hyponatremia.
2. The addition of vaptans to loop diuretics is more effective to elevate serum sodium concentration compared with loop diuretics alone.
3. The addition of vaptans to loop diuretics does not worsen renal function compared with loop diuretics alone.
4. The addition of vaptans to loop diuretics does not show survival benefit compared with loop diuretics alone.
5. The addition of vaptans has the potential to lead to hepatotoxicity in patients with liver cirrhosis.
■ Rationale
Although vaptans have shown effectiveness for correcting SNa in SIAD, heart failure, and liver cirrhosis, the U.S. FDA limited the use of vaptans in liver cirrhosis in 2013 due to hepatic toxicity concerns. The European guideline recommended against treating vaptans in hypervolemic hyponatremia (grade of recommendation 1C). Therefore, we accepted the previous guideline in hyponatremia in liver cirrhosis and did not seek further evidence. We reviewed only patients with heart failure, excluding studies on liver cirrhosis patients.
Including 11 RCTs, one observational study, and two SRs, the guideline found no clinical benefit to the use of vaptans in hypervolemic hyponatremia. However, the quality of the studies varied, the characteristics of enrolled patients were not similar, and most of the RCTs did not distinguish patients with hypervolemia from those with normal volume status. Also, two studies only included patients with liver cirrhosis, and three studies only enrolled patients with heart failure. Thus, it is difficult to conclude that vaptans are superior to loop diuretics. Although vaptans showed a clinical benefit compared with placebo in the two SRs, there was no comparison of vaptans with loop diuretics as a basic therapeutic agent in hypervolemic hyponatremia. Since 2015, various studies have investigated whether additional use of vaptans with loop diuretics could lead to clinical benefit in hyponatremia with heart failure. Only one study compared vaptans and loop diuretics [40], and nearly all studies sought to clarify the effectiveness of additional vaptan use on loop diuretics. Recent studies showed the efficacy of vaptans in patients with chronic kidney disease and heart failure [41,42].
Therefore, this guideline focused on the benefit of additional vaptan use with loop diuretics in hypervolemic hyponatremia with heart failure in terms of survival gain, sodium correction, and conservation of renal function. We reviewed nine RCTs [40–48], five SRs, and several observational studies. All RCTs were conducted in patients with hyponatremic heart failure prescribed tolvaptan 7.5 to 30 mg per day, and allowed furosemide intravenous or oral use. There was no survival benefit of adding tolvaptan on furosemide [40,42,43,46,48]. Renal function decline, which was defined as the increase of serum creatinine more than 0.3 mg/dL per week, was not different between the tolvaptan-added group and furosemide-alone group [40,43,44,47]. Sodium correction for 24 hours was higher when tolvaptan was added to furosemide [42–44].
(Supplement 1| Search strategies—Key question 5.)
(Supplement 2| Study selection flow diagrams—Key question 5.)
(Supplement 3| Summary evidence tables—Key question 5.)
(Supplement 4| Bias risk assessment—Key question 5.)
(Supplement 5| Forest plots—Key question 5.)
(Supplement 6| Clinical evidence profiles [GRADE tables]—Key question 5.)
■ Recommendation considerations
1) Benefits and risks
The U.S. FDA approved tolvaptan as a therapeutic agent for euvolemic or hypervolemic hyponatremia, such as heart failure, liver cirrhosis, or SIAD, in September 2011. However, tolvaptan was found to induce hepatic toxicity in patients with autosomal dominant polycystic kidney disease (ADPKD) in the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3:4 clinical trial [49]. Since the TEMPO trial was conducted in ADPKD patients, the tolvaptan dose was higher (maximum of 120 mg), and the duration of drug use was longer than that in hyponatremia. The U.S. FDA did not set a criterion for tolvaptan dosage. In contrast, Japan was allowed to use low-dose tolvaptan (7.5–15 mg/day) with diuretics (spironolactone/furosemide) to control ascites in patients with liver cirrhosis in 2013. The CPGs for liver cirrhosis 2020 by the Japanese Society of Gastroenterology recommend using tolvaptan 3.75–7.5 mg/day for patients with intractable ascites [50]. The Korean Association for the Study of the Liver CPGs for liver cirrhosis in 2018 did not recommend vaptans with diuretics since the treatment was not effective for controlling ascites and somewhat increased mortality in liver cirrhosis with intractable ascites [51,52].
2) Patient values and preferences
Even though loop diuretics remove overloaded fluid, loop diuretics alone usually fail to correct hyponatremia; high-dose loop diuretics can cause renal insufficiency. Vaptans as a selective aquaretic can be effective to reduce expanded volume and increase SNa at the same time. Adding vaptans to loop diuretics was effective for SNa correction, and does not worsen renal function. This guideline is limited to patients with hyponatremic liver cirrhosis. Although water restriction and albumin could be used in liver cirrhosis with hypervolemic hyponatremia [51], there is a lack of research on whether the additional use of vaptans in patients without ascites will yield clinical benefit.
3) Obstacles, facilitating factors, and measures
Long-term use of vaptans is challenging unless covered by insurance. Korean insurance covers the drug price for limited cases of hyponatremia or patients with ADPKD. Since it is also impossible to prescribe for more than 1 month due to safety concerns such as hepatotoxicity, it is a hassle for patients to visit the hospital every month to receive these drugs. Insurance benefits need to be expanded for various diseases and prescription periods to improve the convenience of tolvaptan use in the clinical field. In addition, it will be necessary to clarify whether tolvaptan alleviates symptoms or reduces mortality when added to essential heart failure medicines.
4) Resource
Korea has two types of tolvaptan; 15 mg and 30 mg. However, the prices of the two drugs are similar, about 10,000 KRW (15 mg, 10,056 KRW and 30 mg, 10,080 KRW). The insurance standard is applied in clinically significant hypervolemic or euvolemic hyponatremia in patients who do not respond to the existing treatment (fluid restriction, hypertonic saline, or diuretics). The total prescription period is limited to 30 days.
Key question 6.
In SIAD patients with moderate to severe hyponatremia, do vaptans improve survival or clinical complications compared with water restriction or loop diuretics?
Recommendation 6
We suggest treatment with vaptans in syndrome of inappropriate antidiuresis (SIAD) patients with moderate to severe hyponatremia.
Conditional recommendation (B), low-quality of evidence
Remarks:
1. There is no direct comparison of vasopressin receptor antagonists with loop diuretics in patients with SIAD. We compared the effects of vaptans with water restriction or placebo.
2. Vaptans have a beneficial effect on normalization of serum sodium in SIAD patients compared with water restriction or placebo.
3. Vaptans do not increase the risk of overcorrection of hyponatremia in SIAD patients compared with water restriction or placebo.
4. Vaptans do not improve survival in SIAD patients compared with water restriction or placebo.
■ Rationale
SIAD is a state of water retention due to a persistent increase in antidiuretic hormone, characterized by hypoosmolar hyponatremia, euvolemia, and high urine osmolality. In patients with SIAD, the standard treatment is the restriction of free water because of water retention [53]. However, there is little evidence that water restriction can effectively treat hyponatremia or does not increase complications such as overcorrection of SNa concentration, ODS, or death. Vaptans correct hyponatremia, effectively causing urinary excretion of free water without increased sodium excretion [54]. However, the European guideline recommends against vasopressin receptor antagonists in SIAD patients without severe or moderately severe symptoms. They emphasized that the safety of vaptans should be considered. First, vaptans can lead to overcorrection of SNa concentration, especially in patients with severe hyponatremia. Second, hepatotoxicity was reported in ADPKD patients on high doses of tolvaptan [10].
Previous studies focused on short-term outcomes such as normalization of SNa or overcorrection. Few studies evaluated the effect of vaptans stratified by volume status: hypervolemia or euvolemia [55,56]. Aggravation of hyponatremia can cause severe symptoms such as poor oral intake, general weakness or altered consciousness, leading to hospitalization. Long-term outcomes were worse in patients who developed repeated symptoms of hyponatremia [36,57].
Placebo or water restriction was used as a control group for vaptans, as there were no studies comparing vaptans with loop diuretics in the previous guidelines or our literature search. Therefore, we reviewed 12 RCTs evaluating the effect of vaptans on sodium correction, survival or complications compared with water restriction or placebo [12,49,58–67]. All RCTs included euvolemic hyponatremia patients: three included only euvolemic hyponatremia, nine included euvolemic or hypervolemic patients. In our meta-analysis, vaptans effectively normalized SNa in euvolemic hyponatremia. Vaptans did not decrease mortality in euvolemic hyponatremia. Although data on complications of vaptans are insufficient, vaptans did not increase the risk of overcorrection of hyponatremia compared with water restriction or placebo. There were few data regarding hepatotoxicity in euvolemic hyponatremia. In conclusion, vaptans can effectively normalize SNa concentration without increased risk of overcorrection or death.
The evidence was low quality and grade (B) in this recommendation because the included participants were not clearly defined as having SIAD, but instead as hypoosmolar hyponatremia with euvolemia or hypervolemia in the included RCTs. However, most euvolemic hyponatremia is SIAD and the diagnostic criteria for SIAD are not clearly defined [68]. Experts agreed that most of included participants might have SIAD. Therefore, we suggest using vaptans in SIAD patients with moderate to severe hyponatremia.
(Supplement 1| Search strategies—Key question 6.)
(Supplement 2| Study selection flow diagrams—Key question 6.)
(Supplement 3| Summary evidence tables—Key question 6.)
(Supplement 4| Bias risk assessment—Key question 6.)
(Supplement 5| Forest plots—Key question 6.)
(Supplement 6| Clinical evidence profiles [GRADE tables]—Key question 6.)
■ Recommendation considerations
1) Benefits and risks
Tolvaptan induced hepatotoxicity in a study investigating the effectiveness and safety among ADPKD patients [69]. The U.S. FDA recommends against tolvaptan in liver disease such as liver cirrhosis. In addition, rapid correction of SNa concentration and the development of hypernatremia are concerns in using tolvaptan. In this study, vaptans did not increase overcorrection significantly compared with placebo or water restriction. Large-scale studies are needed to evaluate complications related to vaptans.
2) Patient values and preferences
SIAD patients with moderate to severe hyponatremia may complain of general weakness, nausea and vomiting, poor oral intake, altered consciousness, hospitalization, and lower quality of life. If consciousness is altered, severe comorbidities such as falls, fractures, and aspiration pneumonia can develop [70]. Therefore, it is important to maintain SNa concentration in SIAD patients. Currently, the standard treatment is a restriction of water intake. It is challenging to maintain SNa concentration with water restriction alone because of low compliance. Vaptans, which lead to selective excretion of free water in urine, can maintain SNa without strict water restriction. However, as summarized in this recommendation, the use of vaptans did not improve mortality.
3) Obstacles, facilitating factors, and measures
The cost of vaptans is an obstacle. Long-term maintenance of vaptans is difficult if not covered by medical insurance. Currently, medical insurance covers vaptan use for 1 month with the diagnostic code for SIAD. After 1 month, medical insurance only covers the cost if additional hyponatremia is documented. Usually SIAD is chronic and requires long-term treatment of hyponatremia. Therefore, insurance coverage of long-term maintenance on vaptans is warranted. Further studies on vaptans and their potential cost reduction by reducing hospitalization, comorbidities, and long-term mortality are needed.
4) Resource
The oral formula of tolvaptan (15 mg, 30 mg) is used in Korea. The cost of one tablet is around 10,000 KRW (10 US dollars). Medical insurance covers tolvaptan for hyponatremia in SIAD if other treatments (water restriction, hypertonic saline, or diuretics) are not effective or not allowed. The total prescription period is limited to 30 days.
Key question 7.
In patients with hypoosmolar hyponatremia, is desmopressin to prevent overcorrection in the treatment of hyponatremia more effective in terms of overcorrection, complications (ODS), and prognosis (survival to discharge) compared with an untreated group?
Recommendation 7
We suggest that desmopressin should be applied individually according to risk factors affecting overcorrection, hypertonic saline therapeutic regimen, and whether to administer dextrose solution during overcorrection in patients with hyponatremia.
Conditional recommendation (B), very low-quality of evidence
Remarks:
1. There is no evidence that administration of desmopressin as a proactive or reactive strategy is effective for preventing overcorrection.
2. Administration of desmopressin in patients with hyponatremia has the potential to increase the incidence of osmotic demyelination syndrome compared to no administration, but drawing a valid conclusion is difficult due to the low level of evidence.
3. Administration of desmopressin for the prevention of overcorrection in hyponatremic patients has the potential to improve survival compared to non-administration, but drawing a valid conclusion is difficult due to the low level of evidence.
■ Rationale
Overcorrection occurring during treatment of patients with hyponatremia is associated with side effects such as ODS. Desmopressin is an antidiuretic hormone that binds to the V2 receptor in the collecting duct and increases the expression of aquaporin channels to increase water reabsorption of urine passing through the collecting duct. A number of studies found that administration of desmopressin can prevent rapid correction of hyponatremia or stabilize SNa correction rate through water reabsorption if it has already been rapidly corrected. The European guideline recommended that 2 µg of intravenous desmopressin be given at intervals of 8 hours or more to prevent rapid correction (grade of recommendation 1D). In addition, they also recommend injecting 10 mL/kg of electrolyte-free water (dextrose solution) for 1 hour in consideration of urine volume and fluid balance (grade of recommendation 1D). However, there have been no prospective studies on this recommendation. In two retrospective observational studies cited in the guidelines, SNa concentration was corrected using desmopressin or electrolyte-free water to the target of 12 mmol/L within 24 hours and less than 18 mmol/L within 48 hours when overcorrection occurred [71]. In one of these retrospective studies, the proactive strategies in which hypertonic fluid and desmopressin were concurrently administered increased SNa concentration stably, and SNa concentration was maintained within the target range for 24 and 48 hours [72]. However, the quality of the studies included in the guidelines varied, the criteria for classification of hyponatremia among the study subjects varied, and there was no comparative study in which patients not using desmopressin were included as a control group. Eighty patients using desmopressin were classified into three strategies in one SR of desmopressin use for hyponatremia in 2015 [73]. The proactive strategy was based on initial SNa concentration, with desmopressin administered before concentration changes of SNa. In the reactive strategy, desmopressin was administered according to an increase in the concentration of SNa or urine output. In the rescue strategy, desmopressin was administrated to re-lower SNa concentration in case of overcorrection. However, final conclusions could not be drawn on the optimal strategy for administration of desmopressin for hyponatremia due to limitations of the study design and sample size.
In this guideline, we evaluated one SR study and three observational studies on whether the administration of desmopressin for hyponatremia has additional benefit in the prevention of overcorrection, complications (ODS), and prognosis (survival to discharge) compared to the non-administered group [71,73–75]. As a result of our analysis, overcorrection prevention did not differ significantly when comparing the group with and without use of desmopressin (proactive and reactive strategies). When the desmopressin use group and the non-desmopressin group were compared, including proactive, reactive, and rescue therapy, the incidence of ODS was higher in the group using desmopressin, but ODS occurred in only one or two cases, and there was a possibility of selection bias. When comparing the survival to discharge of the groups administered and not administered desmopressin (proactive, reactive and rescue strategies), survival rate was significantly higher in the desmopressin use group. However, a larger sample size and prospective studies are needed to determine the optimal strategy for desmopressin administration in hyponatremia.
(Supplement 1| Search strategies—Key question 7.)
(Supplement 2| Study selection flow diagrams—Key question 7.)
(Supplement 3| Summary evidence tables—Key question 7.)
(Supplement 4| Bias risk assessment—Key question 7.)
(Supplement 5| Forest plots—Key question 7.)
(Supplement 6| Clinical evidence profiles [GRADE tables]—Key question 7.)
■ Recommendation considerations
1) Benefits and risks
We analyzed the benefits of desmopressin with regard to overcorrection, ODS, and death. The risk of overcorrection is not equal among all patients, and there is an overcorrection risk group [11,76–79]. Data on ODS or death occurring during the correction of hyponatremia are extremely rare, so large-scale clinical trials are necessary to evaluate benefits and risks. There are many limitations to setting a ODS as an outcome, because ODS is diagnosed at a different rate depending on the severity of symptoms, and retrospective studies have the possibility of selection bias. Therefore, we judged that the evidence was insufficient to recommend the general use of desmopressin based data obtained up to the present point. However, there is no evidence to limit the use of desmopressin in some groups at risk of overcorrection, so we withheld judgment the use of desmopressin.
2) Patient values and preferences
High risk groups for overcorrection have been reported to include alcoholism, malnutrition, advanced liver disease, hypokalemia, and SNa of ≤105 mmol/L [11,76–79]. In these groups, not only overcorrection but also safety indicators such as ODS and death are highly likely to occur, so it is necessary to prepare for this. However, we recommend tailoring treatment to the patients since there are various factors affecting overcorrection such as patient risk factors, administration regimen or rate of hypertonic saline [17,80,81], and electrolyte-free water treatment.
3) Obstacles, facilitating factors, and measures
Although the risk of overcorrection in hyponatremia is well known, the level of evidence for this is very poor. More prospective clinical trials are needed to obtain evidence for a treatment regimen after its occurrence.
4) Resource
None.
Key question 8.
In hypoosmolar hyponatremia among patients with cerebral diseases, is saline infusion effective in correcting SNa concentration and preventing complications compared to placebo?
Recommendation 8
We consider it reasonable that treatment with hypertonic or isotonic saline infusion, oral sodium chloride, or fludrocortisone for the correction of hypoosmolar hyponatremia should be individualized among patients with cerebral diseases.
Expert consensus
Remarks:
1. The causes of hypoosmolar hyponatremia among patients with cerebral diseases are diverse, and include syndrome of inappropriate antidiuresis, cerebral salt wasting, and insufficient cortisol secretion.
2. There is insufficient evidence that hypoosmolar hyponatremia in patients with cerebral diseases can be effectively corrected with a crystalloid solution, including normal saline.
■ Rationale
Hyponatremia occurs very frequently in patients with various cerebral diseases such as traumatic brain injury, intracranial or subarachnoid hemorrhage, brain tumor, brain surgery, cerebral infarction, and meningitis. The incidence of hyponatremia in traumatic brain injury patients has been reported to be 27% to 51% [82,83], 40% to 45% in cerebral infarction patients [84], 14% to 63% in subarachnoid hemorrhage [84,85], and 15% to 20% in brain tumor patients [86]. Various factors such as SIAD, CSW, and insufficient secretion of cortisol are major causes of hyponatremia in cerebral diseases. The most common cause of hyponatremia in patients with cerebral disease is SIAD, accounting for approximately 62%, and volume deficit or CSW accounted for about 30% [86].
Concomitant hyponatremia in patients with cerebral disease is closely related to the deterioration of patient condition. Therefore, appropriate treatment depending on the cause of hyponatremia is highly recommended [11]. However, in a clinical setting, it is not easy to accurately determine the cause based on patient volume status and it may require several hours or days to complete diagnostic tests and evaluations to determine the cause. Therefore, the CPG Committee sought to suggest appropriate treatment guidelines for hypoosmolar hyponatremia in patients with various cerebral diseases. We searched Ovid MEDLINE, Embase, Cochrane Library, and KMbase, and found a total of 72 research papers through additional manual searches. We selected 66 documents excluding duplicates and reviewed 13 original texts. We could not find any documents that suitably addressed this key question. Therefore, an expert consensus was made by organizing the results of related research with the existing CPGs.
The American guideline suggested that in the case of hyponatremia in patients with cerebral disease, treatment such as normal saline, oral salt supplementation, hypertonic saline, and fludrocortisone may be considered [11]. In general, treatment guidelines recommend water restriction or hypertonic saline depending on the severity of hyponatremia in SIAD. In addition, volume depletion is common in patients with various cerebral diseases [87]. In particular, volume deficit (body fluid deficiency) accompanying CSW can result in hyponatremia. The occurrence of cerebral infarction and other neurological complications increases when water restriction is implemented in patients with cerebral disease [88,89]. Therefore, in the case of hyponatremia accompanying these cerebral diseases, clinicians should avoid volume depletion through water restriction [90].
Hyponatremia accompanied by neurological symptoms related to hyponatremia can be corrected through hypertonic saline to prevent the progression of neurological complications. However, it should be treated cautiously by controlling the rate of correction to avoid overcorrection in accordance with the general principles of hyponatremia correction rate. Asymptomatic hypoosmolar hyponatremia occurring in patients with cerebral diseases can be initially corrected by preferentially using isotonic crystalloid solution including normal saline, unless volume depletion is clearly excluded by clinical judgment. In the case of asymptomatic hypoosmolar hyponatremia that does not improve despite administering isotonic crystalloid solution, such as normal saline, evaluation and tests for differential diagnosis may be performed, and concomitant salt supplementation, such as hypertonic saline or oral salt may be considered. In the case of CSW, hyponatremia can be corrected with a mineralocorticoid such as fludrocortisone. Hasan et al. [91] demonstrated the effectiveness of fludrocortisone treatment for the prevention of renal salt excretion and volume status decrease in 91 patients with subarachnoid hemorrhage, which is commonly accompanied by CSW, through a RCT. Misra et al. [92] conducted a RCT on 38 hyponatremic patients with tuberculous meningitis due to CSW and showed that fludrocortisone treatment can correct hyponatremia earlier than normal saline. Further evidence is needed for specific recommendations in hyponatremia patients with cerebral diseases.
(Supplement 1| Search strategies—Key question 8.)
(Supplement 2| Study selection flow diagrams—Key question 8.)
(Supplement 3| Summary evidence tables—Key question 8.)
(Supplement 4| Bias risk assessment—Key question 8.)
(Supplement 5| Forest plots—Key question 8.)
(Supplement 6| Clinical evidence profiles [GRADE tables]—Key question 8.)
■ Recommendation considerations
1) Benefits and risks
Several observational studies have reported that a decrease in volume status increases the risk of complications such as cerebral infarction in patients with cerebral diseases [88,89]. There are few studies comparing the effects of normal saline with controls in hyponatremic patients with cerebral diseases. Administration of hypertonic or isotonic saline infusion, oral sodium chloride, or fludrocortisone to prevent deterioration of hyponatremia and other neurological complications including cerebral infarction might have more benefits than risks, such as exacerbation of hyponatremia.
2) Patient values and preferences
In the relevant clinical situation, it is inappropriate to make a treatment decision in consideration of patient preferences, as treatment according to medical judgment takes priority.
3) Obstacles, facilitating factors, and measures
There are no predictable obstacles that may arise when this recommendation is applied to clinical settings.
4) Resource
Treatment including hypertonic or isotonic saline infusion, oral sodium chloride, or fludrocortisone can be used without limitation in most domestic medical environments.
Key question 9.
In hospitalized pediatric patients under the age of 18 years, is the administration of isotonic fluids as maintenance fluids effective for preventing hyponatremia without increasing the risk of hypernatremia compared to hyponatremic fluids?
Recommendation 9
1. To prevent hyponatremia, we recommend the administration of isotonic fluids as maintenance fluid therapy in hospitalized pediatric patients over 1 month and under 18 years of age.
Strong recommendation (A), high-quality evidence
2. There are insufficient data to make a recommendation regarding administrating isotonic fluids as maintenance fluid therapy to prevent hyponatremia in neonates because of the risk of hypernatremia.
Inconclusive (I), moderate-quality evidence
Remarks:
1. In maintenance fluid therapy for children and adolescents over 1 month and under 18 years of age, the administration of isotonic fluid is effective for preventing the development of hyponatremia and has similar risk of hypernatremia compared to the administration of hypotonic fluids.
2. In maintenance fluid therapy for neonates less than 1 month old, the administration of isotonic fluid is effective for preventing the development of hyponatremia and leads to a higher risk of developing hypernatremia compared to the administration of hypotonic fluids.
■ Rationale
Traditionally, hypotonic solutions based on the Holliday-Segar formula have been used as maintenance fluids in hospitalized pediatric patients under the age of 18 years. However, the development of hyponatremia associated with hypotonic solution administration and related neurologic complications and death have been continuously reported. In addition, children are more likely to develop severe symptoms associated with hyponatremia because the brain is relatively large compared to the skull in children. Consequently, there has been controversy over the composition of optimal maintenance fluid. In 2018, the American Academy of Pediatrics recommended an isotonic solution as a maintenance fluid for pediatric patients over 1 month old by integrating evidence from 17 RCTs and seven SRs (grade of recommendation 1A) [93]. In the 2020 revised NICE (National Institute for Health and Care Excellence) guidelines, isotonic solutions were recommended as maintenance fluids in children, including term neonates 8 days of age or older [94]. However, RCT studies have shown inconsistent results.
In this guideline, we performed a meta-analysis by synthesizing 18 RCTs (16 RCTs for children over 1 month and two RCTs for newborns) [95–112] and seven SRs [113–115]. We sought to examine whether the administration of isotonic fluid compared to hypotonic fluids reduced the incidence of hyponatremia without increasing the risk of hypernatremia during maintenance fluid therapy in pediatric patients including newborns. In 18 RCTs, normal saline or Ringer’s lactate solution as an isotonic solution and 0.20% to 0.45% saline as a hypotonic solution were administered to hospitalized pediatric patients. In most studies, 5% dextrose solution was added to the maintenance fluid. A total of 3,231 patients were included in 16 RCT studies of children 1 month and older, including patients who were hospitalized for surgery or were admitted to the intensive care unit, and those who were hospitalized for pneumonia or central nervous system infection. Of these, isotonic solutions were used in 1,608 patients and hypotonic solutions were used in 1,623 patients as maintenance fluid. The incidence of hyponatremia in patients using isotonic solution as maintenance treatment was significantly lower than in the group using hypotonic solution as maintenance treatment (OR, 0.32; 95% CI, 0.24–0.43; p < 0.001). Although hypernatremia was increased in patients administered isotonic solutions in some studies [95–98,101,105], there was no significant difference in a meta-analysis between isotonic fluid and hypotonic fluid (OR, 1.67; 95% CI, 0.92–3.04; p = 0.09). In two non-RCTs, the incidence of hyponatremia had a tendency to be low in patients administered isotonic fluids; however, this was not statistically significant (OR, 0.54; 95% CI, 0.28–1.02; p = 0.05), and the incidence of hypernatremia in patients receiving isotonic fluids was not significantly different from that in patients receiving hypotonic fluids (OR, 1.25; 95% CI, 0.73–2.13; p = 0.58). Two RCTs enrolled a total of 144 neonates, including premature babies aged 34 weeks or older and full-term neonates. Of these, 73 patients received isotonic fluid and 71 received 0.15% to 0.20% hypotonic fluid. These studies reported a significantly lower incidence of hyponatremia in patients receiving isotonic fluids (OR, 0.11; 95% CI, 0.03–0.35; p < 0.001) than in patients receiving hypotonic fluids. However, the incidence of hypernatremia was significantly higher in patients receiving isotonic fluids than in patients receiving hypotonic fluids (OR, 8.24; 95% CI, 1.84–36.91; p < 0.001) [100,104].
(Supplement 1| Search strategies—Key question 9.)
(Supplement 2| Study selection flow diagrams—Key question 9.)
(Supplement 3| Summary evidence tables—Key question 9.)
(Supplement 4| Bias risk assessment—Key question 9.)
(Supplement 5| Forest plots—Key question 9.)
(Supplement 6| Clinical evidence profiles [GRADE tables]—Key question 9.)
■ Recommendation considerations
1) Benefits and risks
Overall, the use of isotonic fluids as a maintenance fluid for adolescents and children over 1 month and under 18 years of age has more benefits than risks. However, in neonates less than 1 month old, the incidence of hypernatremia was significantly higher in the isotonic fluid-treated group than in the hypotonic fluid-treated group. In the neonatal period, total body water and electrolyte distribution differs from that during infancy and childhood. In addition, the neonatal kidney is less efficient in excreting acute sodium or water load than the kidney of an infant or a child [116]. The urine concentrating ability is impaired in infants and newborns. There is insufficient evidence to draw conclusions on the use of isotonic fluids as a maintenance fluid in newborns less than 1 month old.
2) Patient values and preferences
Because doctors usually select maintenance fluids, it is impractical to consider patient values and preferences. Several pieces of evidence suggest that using an isotonic fluid as a maintenance fluid is beneficial.
3) Obstacles, facilitating factors, and measures
We expect no obstacles to accepting this recommendation. Therefore, doctors who treat children and adolescents should encourage the use of isotonic fluids as a maintenance fluid, through expanded education.
4) Resource
Fluid treatment is covered under medical insurance in Korea; thus, additional resources are not required.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.33.666).
Notes
Conflict of interest
All authors have no conflicts of interest to declare.