| Kidney Res Clin Pract > Volume 41(6); 2022 > Article |
|
Abstract
Background
Methods
Results
Notes
Conflicts of interest
Tae-Hyun Yoo is the Editor-in-Chief of Kidney Research and Clinical Practice and was not involved in the review process of this article. All authors have no other conflicts of interest to declare.
Authors’ contributions
Conceptualization, Project administration, Resources, Software: SCK, JTP
Data curation: SCK, HBK, HWK, YSJ, JTP
Formal analysis: SCK, HBK, JTP
Investigation, Methodology: SCK, HBK, HWK, JTP
Supervision: SHH, THY, SWK
Visualization: SCK, JTP
Writing–original draft: SCK, JTP
Writing–review & editing: SCK, JTP
All authors read and approved the final manuscript.
Acknowledgments
Supplementary Materials
Figure 1.
Flow diagram of the study.
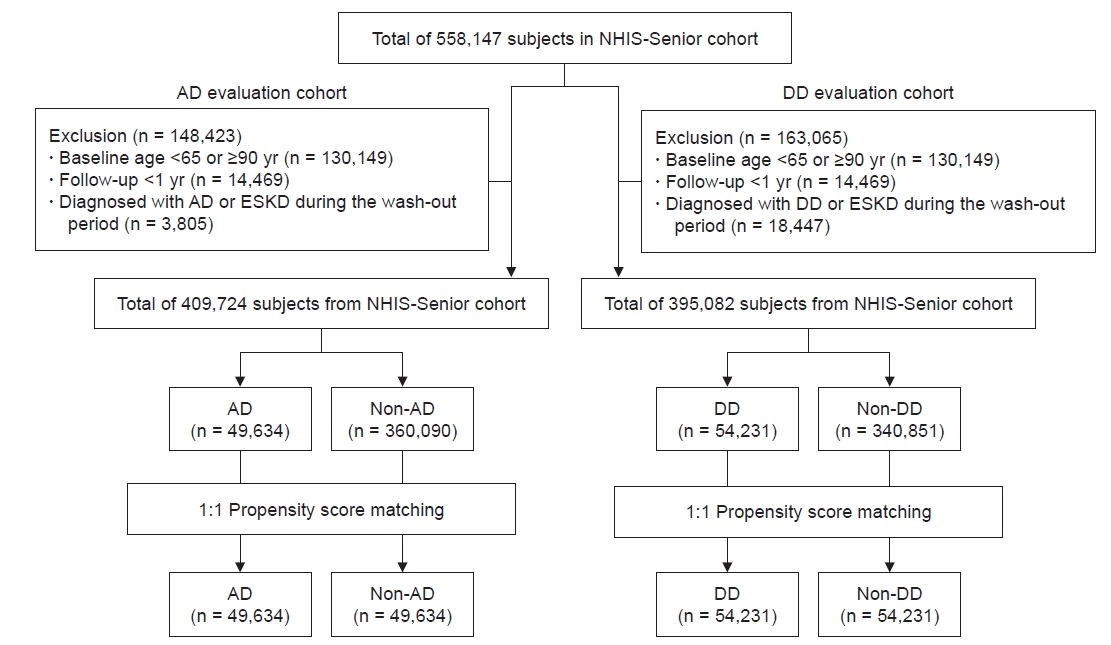
Figure 2.
Schematic depiction of the study design.
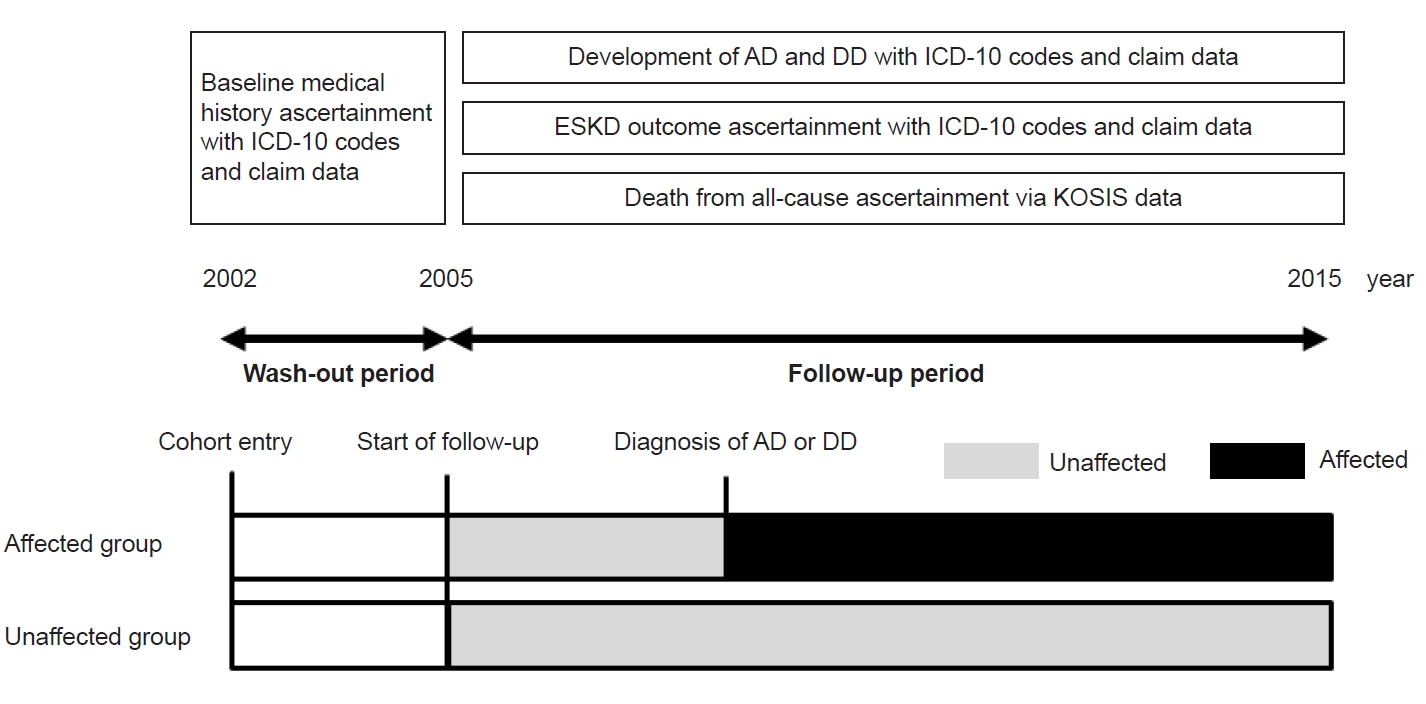
Figure 3.
Cumulative incidence curves of incident end-stage kidney disease in propensity score-matched subjects diagnosed with AD (A) or DD (B).
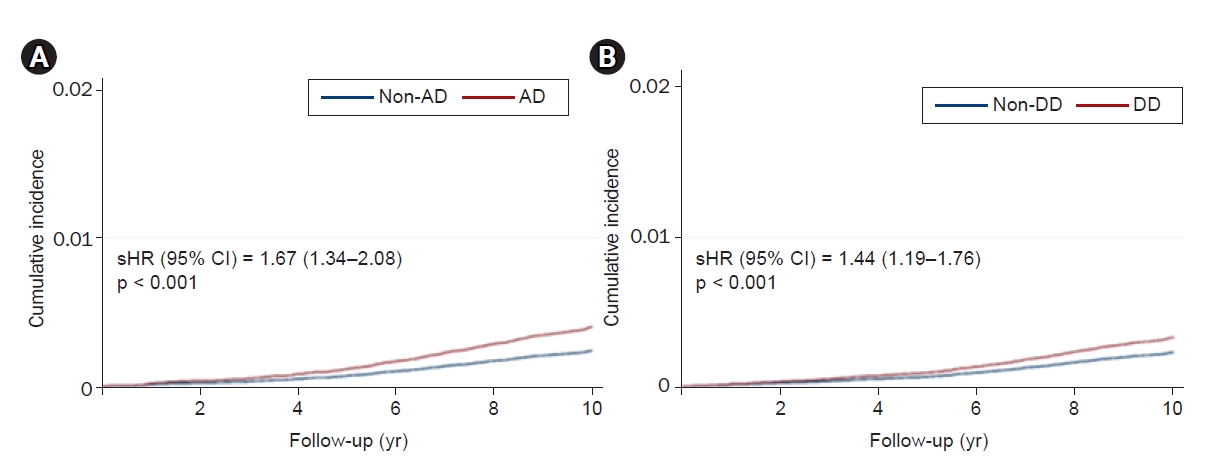
Table 1.
| Variable |
AD evaluation cohort |
DD evaluation cohort |
||||
|---|---|---|---|---|---|---|
| Non-AD (n = 49,634) | AD (n = 49,634) | SMD | Non-DD (n = 54,231) | DD (n = 54,231) | SMD | |
| Demographic data | ||||||
| Age (yr) | 74 (69–78) | 74 (69–78) | 0.002 | 70 (67–75) | 70 (67–75) | 0.002 |
| Age at AD/DD diagnosis (yr)a | 80 (76-85) | 76 (73–80) | ||||
| Age at ESKD diagnosis (yr)a | 78 (74–82) | .79.5 (76–83) | 77 (73–81) | 78 (74–81) | ||
| Time from AD/DD to ESKD (mo)a | .21.0 (8.7–39.9) | .31.8 (9.5–51.4) | ||||
| Male sex | 14,799 (29.8) | 14,924 (30.1) | 0.01 | 18,319 (33.8) | 18,853 (34.8) | 0.02 |
| Follow-up duration (yr) | 10 (9–10) | 10 (9–10) | 0.02 | 10 (10–10) | 10 (10–10) | 0.004 |
| Urban residence | 25,423 (51.2) | 25,720 (51.8) | 0.01 | 29,526 (54.4) | 29,510 (54.4) | 0.001 |
| Income, highest tertile | 14,503 (29.2) | 14,866 (30.0) | 0.02 | 17,489 (32.2) | 17,752 (32.7) | 0.01 |
| Comorbidity | ||||||
| Hypertension | 16,730 (33.7) | 17,182 (34.6) | 0.02 | 21,113 (38.9) | 21,511 (39.7) | 0.02 |
| Diabetes mellitus | 5,062 (10.2) | 5,447 (11.0) | 0.02 | 5,625 (10.4) | 5,866 (10.8) | 0.01 |
| Dyslipidemia | 4,183 (8.4) | 4,533 (9.1) | 0.02 | 6,712 (12.4) | 7,056 (13.0) | 0.02 |
| Chronic kidney disease | 305 (0.6) | 301 (0.6) | 0.002 | 352 (0.6) | 394 (0.7) | 0.003 |
| Myocardial infarction | 662 (1.3) | 774 (1.6) | 0.02 | 841 (1.6) | 1,015 (1.9) | 0.02 |
| Congestive heart failure | 2,495 (5.0) | 2,773 (5.6) | 0.02 | 2,906 (5.4) | 3,150 (5.8) | 0.02 |
| Peripheral arterial disease | 915 (1.8) | 896 (1.8) | 0.002 | 2,772 (5.1) | 3,005 (5.5) | 0.02 |
| Cerebrovascular disease | 2,876 (5.8) | 2,968 (6.0) | 0.02 | 3,636 (6.7) | 3,861 (7.1) | 0.02 |
| Malignancy | 1,576 (3.2) | 1,618 (3.3) | 0.004 | 2,061 (3.8) | 2,296 (4.2) | 0.02 |
| Chronic liver disease | 2,429 (4.9) | 2,518 (5.1) | 0.01 | 3,661 (6.8) | 3,840 (7.1) | 0.01 |
| COPD | 5,695 (11.5) | 6,012 (12.1) | 0.02 | 7,283 (13.4) | 7,549 (13.9) | 0.01 |
| Osteoporosis | 7,003 (14.1) | 7,160 (14.4) | 0.01 | 8,898 (16.4) | 9,055 (16.7) | 0.01 |
| Medication history | ||||||
| RAAS blocker | 9,853 (19.9) | 10,337 (20.8) | 0.02 | 12,666 (23.4) | 12,924 (23.8) | 0.01 |
| DHP-CCB | 12,827 (25.8) | 12,975 (26.1) | 0.02 | 16,079 (29.6) | 16,225 (29.9) | 0.02 |
| Statin | 3,749 (7.6) | 4,047 (8.2) | 0.02 | 5,716 (10.5) | 6,104 (11.3) | 0.02 |
| Diuretics | 4,067 (8.2) | 4,322 (8.7) | 0.01 | 5,466 (10.1) | 5,765 (10.6) | 0.01 |
Data are expressed as median (interquartile range) or number (%).
AD, Alzheimer disease; COPD, chronic obstructive pulmonary disease; DD, depressive disorder; DHP-CCB, dihydropyridine calcium channel blocker; ESKD, end-stage kidney disease; RAAS, renin-angiotensin aldosterone system; SMD, standardized mean difference.
Table 2.
| Variable | Event rate per 1,000 person-years | Crude HR, sHR (95% CI) | p-value | Adjusted HRa, sHR (95% CI) | p-value |
|---|---|---|---|---|---|
| AD evaluation cohort | |||||
| Non-AD | 0.36 | Reference | Reference | ||
| AD | 1.17 | 1.86 (1.50–2.30) | <0.001 | 1.67 (1.34–2.08) | <0.001 |
| DD evaluation cohort | |||||
| Non-DD | 0.36 | Reference | Reference | ||
| DD | 0.91 | 1.75 (1.43–2.13) | <0.001 | 1.44 (1.19–1.76) | <0.001 |
AD, Alzheimer disease; CI, confidence interval; DD, depressive disorder; HR, hazard ratio; sHR, subdistribution HR.
a Adjusted for clinical variables used to estimate propensity scores (listed in Table 1).
Table 3.
| Subgroup | No. of patients | Crude HR, sHR (95% CI) | p-value | Adjusted HRa, sHR (95% CI) | p-value | p for interaction |
|---|---|---|---|---|---|---|
| AD evaluation cohort | ||||||
| Sex | 0.71 | |||||
| Male | 29,723 | |||||
| Non-AD | 14,799 | Reference | Reference | |||
| AD | 14,924 | 1.63 (1.20–2.23) | 0.002 | 1.56 (1.14–2.14) | 0.006 | |
| Female | 69,545 | |||||
| Non-AD | 34,835 | Reference | Reference | |||
| AD | 34,710 | 2.09 (1.55–2.81) | <0.001 | 2.07 (1.54–2.80) | <0.001 | |
| Residential area | 0.81 | |||||
| Urban residence | 51,143 | |||||
| Non-AD | 25,423 | Reference | Reference | |||
| AD | 25,720 | 1.85 (1.42–2.42) | <0.001 | 1.82 (1.39–2.38) | <0.001 | |
| Rural residence | 48,125 | |||||
| Non-AD | 24,211 | Reference | Reference | |||
| AD | 23,914 | 1.82 (1.27–2.61) | 0.001 | 1.92 (1.33–2.76) | <0.001 | |
| Disease onset | 0.99 | |||||
| Non-AD | 49,634 | Reference | Reference | |||
| Early onset ADb | 25,967 | 1.92 (1.52–2.43) | <0.001 | 1.68 (1.32–2.12) | <0.001 | |
| Late onset ADb | 23,667 | 1.07 (0.79–1.46) | 0.65 | 1.87 (1.31–2.67) | 0.001 | |
| DD evaluation cohort | ||||||
| Sex | 0.67 | |||||
| Male | 37,172 | |||||
| Non-DD | 18,319 | Reference | Reference | |||
| DD | 18,853 | 1.86 (1.42–2.45) | <0.001 | 1.67 (1.27–2.19) | <0.001 | |
| Female | 71,290 | |||||
| Non-DD | 35,912 | Reference | Reference | |||
| DD | 35,378 | 1.62 (1.20–2.18) | 0.002 | 1.53 (1.15–2.05) | 0.004 | |
| Residential area | 0.15 | |||||
| Urban residence | 59,036 | |||||
| Non-DD | 29,526 | Reference | Reference | |||
| DD | 29,510 | 1.52 (1.18–1.96) | 0.001 | 1.43 (1.11–1.84) | 0.006 | |
| Rural residence | 49,426 | |||||
| Non-DD | 24,705 | Reference | Reference | |||
| DD | 24,721 | 2.22 (1.61–3.07) | <0.001 | 1.99 (1.40–2.76) | <0.001 | |
| Disease onset | 0.84 | |||||
| Non-DD | 54,231 | Reference | Reference | |||
| Early onset DDb | 28,786 | 1.58 (1.27–1.96) | <0.001 | 1.52 (1.22–1.89) | <0.001 | |
| Late onset DDb | 25,445 | 1.27 (1.14–1.80) | 0.03 | 1.43 (1.05–1.95) | 0.02 |
AD, Alzheimer disease; CI, confidence interval; DD, depressive disorder; HR, hazard ratio; sHR, subdistribution HR.
a Adjusted for clinical variables used to estimate propensity scores (listed in Table 1) and competing risk of all-cause death.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Shin Chan Kang

https://orcid.org/0000-0002-6507-2676Hee Byung Koh

https://orcid.org/0000-0002-4510-2823Hyung Woo Kim

https://orcid.org/0000-0002-6305-452XYoung Su Joo

https://orcid.org/0000-0002-7890-0928Seung Hyeok Han

https://orcid.org/0000-0001-7923-5635Tae-Hyun Yoo

https://orcid.org/0000-0002-9183-4507Shin-Wook Kang

https://orcid.org/0000-0002-5677-4756Jung Tak Park

https://orcid.org/0000-0002-2325-8982 - Related articles
-
The impact of severe depression on the survival of older patients with end-stage kidney disease



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















