Nitric oxide-inhibited chloride transport in cortical thick ascending limbs is reversed by 8-iso-prostaglandin-F2α
Article information
Abstract
Background
Sodium chloride (NaCl) reabsorption in the cortical thick ascending limb (cTAL) is regulated by opposing effects. Nitric oxide (NO) inhibits NaCl reabsorption while 8-iso-prostaglandin-F2α (8-iso-PGF2α) stimulates it. Their interaction has not been evaluated in the cTAL. Because 8-iso-PGF2α has considerable stability while NO is a free radical with a short half-life, we hypothesized that, in the cTAL, the inhibition of NaCl absorption will be reversed by 8-iso-PGF2α.
Methods
Chloride absorption (JCl) was measured in isolated perfused cTALs and whether the activation of protein kinase A (PKA) is required for this interaction. Since cyclic adenosine monophosphate (cAMP) is a major messenger for the 8-iso-PGF2α signaling cascade, and NO inhibits JCl by decreasing cAMP bioavailability, we measured 8-iso-PGF2α–stimulated cAMP in the presence of sodium nitroprusside (SNP).
Results
The NO donor, SNP (10–6 M), decreased JCl by 41%, while luminal 8-iso-PGF2α (100 μM) increased JCl to 315 ± 46 pmol/min/mm (p < 0.003), reversing the effects of the NO donor. SNP inhibited JCl, 8-iso-PGF2α failed to increase JCl in the presence of H89. Basal cAMP was 56 ± 13 fmol/min/mm, in the presence of SNP 57 ± 6 fmol/min/mm, and 8-iso-PGF2α increased it to 92 ± 2 fmol/min/mm (p < 0.04).
Conclusion
We concluded that 1) NO-induced inhibition of JCl in the cTAL can be reversed by 8-iso-PGF2α, 2) 8-iso-PGF2α and NO interaction requires PKA to control JCl, and 3) in the presence of NO, 8-iso-PGF2α continues to stimulate JCl because NO cannot reverse 8-iso-PGF2α-stimulated cAMP level.
Introduction
The nephron segment, cortical thick ascending limb (cTAL) regulates sodium chloride (NaCl) reabsorption as one of the most involved segments in NaCl retention states. In normal conditions, absorption of NaCl is balanced with its excretion, maintaining Na balance. This homeostasis is the final result of the action of molecules increasing and decreasing transport. However, in pathologic conditions, such as congestive heart failure, chronic kidney disease, hypertension, cirrhosis, and other diseases, Na retention is greater than its excretion, leading to a positive Na imbalance. This Na overload occurs because Na retention factors overcome the actions of molecules that increase its excretion, resulting in resistance to natriuretic effects. Understanding the interaction of the autacoids at the different nephron segments, especially at the cTAL, would help to elucidate such deleterious conditions and find strategies to manage them.
Previously, we reported that 8-iso-prostaglandin-F2α (8-iso-PGF2α) increases Na+ and Cl– reabsorption at the cTAL [1]. This effect, in vivo, would increase Na balance, inducing a blood pressure elevation that could be displayed as hypertension, congestive heart failure, cirrhosis, chronic kidney disease, or other edematous states. On the other hand, nitric oxide (NO) inhibits Na+ reabsorption at the cTAL, and this effect, in vivo, would induce diuresis and natriuresis, a mechanism that would decrease the described Na gain [2,3]. Despite the clear understanding of the importance of these effects, the interaction of NO and 8-iso-PGF2α in the cTAL has not been evaluated to elucidate their outcomes.
Na+ regulation has been previously reported at several nephron segments mediated by agents such as NO and angiotensin II in the cTAL [4,5], NO and arginine vasopressin at the cortical collecting duct level [6], and other segments [7]. The final effect of the interaction between NO and 8-iso-PGF2α is based on the intracellular activation of different regulators. Actually, whereas NO inhibits NaCl reabsorption by increasing intracellular cyclic guanosine monophosphate (cGMP) and decreasing cyclic adenosine monophosphate (cAMP) levels [7], 8-iso-PGF2α increases cAMP. The final effect will be elicited when one cyclic nucleotide preponderantly represses the other. When attempting to understand the crosstalk regulation of cyclic nucleotide concentrations, one must consider that such regulation can occur at the level of cyclic nucleotide synthesis, phosphodiesterase-mediated degradation, or even cellular efflux. Thus, we hypothesized that, in cTAL, persistent effects of 8-iso-PGF2α on Na reabsorption prevail over the shorter-acting NO effects, and protein kinase A (PKA) activation is required for such interaction.
Methods
Animals
Male New Zealand white rabbits (700–1,200 g) were housed for 3 to 7 days before the study for acclimatization. The experiments were approved by the Institutional Animal Care and Use Committee of the J. Robert Cade Foundation (No. FJRC #2009) in Córdoba, Argentina. All animals were housed and handled according to the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Preparation and dissection of the cortical thick ascending limb of Henle
Animals were anesthetized with sodium thiopental (30 mg/kg, intravenously) and xylazine (20 mg/kg, intramuscularly) plus ketamine (50 mg/kg, intramuscularly). The kidney was exposed and bathed in ice-cold saline and then excised and cut into coronal slices along the longitudinal axis. The slices were placed in oxygenated physiological saline in a temperature-regulated chamber at 4°C. The cTAL of Henle was dissected from the medullary ray under a stereomicroscope as previously described [6,8].
Perfusion of thick ascending limbs
cTALs in a temperature-regulated chamber were perfused between concentric glass pipettes at 37°C as described [6]. The basolateral bath was exchanged at a rate of 0.5 mL/min. A total of five to eight tubules were analyzed during each protocol. Time-control experiments were conducted to ensure the stability of tubular transport. Chloride absorption (JCl) was measured as previously described [6,9]. cTALs were perfused and equilibrated for 20 minutes, and basal Cl absorption was calculated two to three times. Tested compounds, including 8-iso-PGF2α, were either added to or removed from the lumen or bath as indicated. After a 20-minute equilibration period, tubular fluid was again collected 2 or 3 times.
Chloride concentrations in the perfusate and collected fluid
Chloride concentrations were measured by microfluorometry [10]. Because water is not reabsorbed by the thick ascending limb, JCl was calculated by the following equation: JCl = CR × (C0Cl – C1Cl), where CR = collection rate normalized/tubule length, C0Cl = chloride concentration in the perfusion solution, and C1Cl = chloride concentration in the collected fluid.
Cyclic adenosine monophosphate
cAMP was measured as previously described [1]. Briefly, tubules were isolated and incubated in 95 µL perfusion solution containing 1-mM 3-isobutyl-1-methylxanthine at 37°C for 10 minutes before adding 5-µL medium containing inhibitors or hormones. The reaction was stopped 30 minutes later with methanol (100 µL), and cAMP and cGMP levels were measured by an enzyme immunoassay (EIA; Cayman Chemical, Ann Arbor, MI, USA). The samples were centrifuged, the supernatant was transferred to another tube and dried in a Savant centrifugal vacuum concentrator, and the pellet was reconstituted in 110-µL sodium acetate buffer. The cAMP standard was treated the same way. The results were expressed as fmol/min/mm tubule length.
Statistics
Data were analyzed by paired and unpaired Student t tests and one-way analysis of variance, and results with p < 0.05 were significant. Results were expressed as mean ± standard error of the mean.
Results
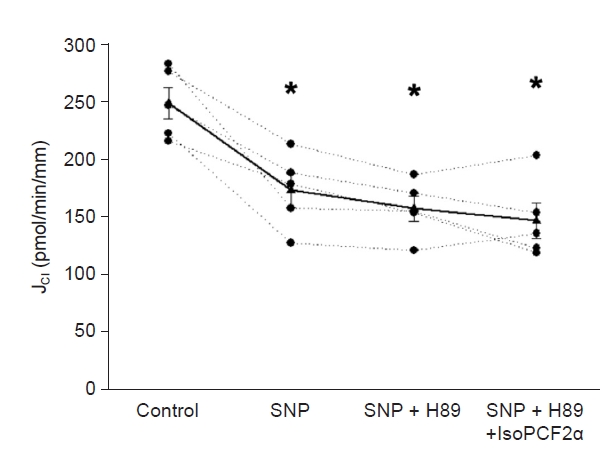
Since the 8-iso-PGF2α/NO interaction in cTAL is uncertain, we chose a maximum NO bioavailability model. As described, the micro-isolated rabbit cTALs were transferred to a temperature-regulated chamber and perfused between concentric glass pipettes at 37°C [6] with the NO donor sodium nitroprusside (SNP) in a concentration that decreases transport by 40% as endogenous NO is inhibited in this nephron segment [11]. Before adding SNP to the bath, JCl was 333 ± 35 pmol/min/mm. SNP (1 mM) in the bath reduced JCl to 195 ± 26 pmol/min/mm (p = 0.01 vs. basal; n = 5) (Fig. 1), a 41% decrease, and remained inhibited for the rest of the experiment (196 ± 22 pmol/min/mm). In vivo, this reduction in JCl should increase diuresis. In time-control experiments, JCl remained constant throughout the experimental period.
SNP inhibits JCl; nitric oxide decreases JCl in the cTAL.
Basal JCl was 333.5 ± 35.2 pmol/min/mm and the addition of SNP (1 mM) decreased it to 195.9 ± 26.1 pmol/min/mm (*p = 0.01 vs. basal; n = 5, one-way analysis of variance [Holm-Sidak test]) and remained decreased during the experimental time (196.5 ± 22.3 pmol/min/mm).
cTAL, cortical thick ascending limb; JCl, chloride absorption; SNP, sodium nitroprusside.
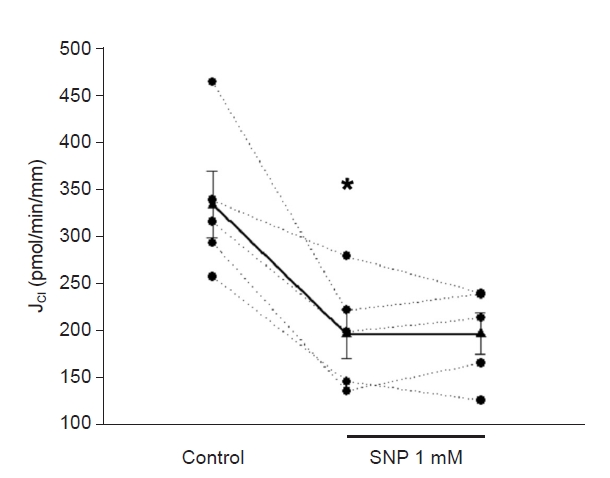
Next, we tested whether 8-iso-PGF2α stimulates JCl in the presence of SNP. Basal JCl was 330 ± 18 pmol/min/mm; when SNP (1 mM) was added to the bath, JCl decreased to 191 ± 19 pmol/min/mm. After adding 8-iso-PGF2α (100 μM) to the lumen, JCl increased to 346 ± 39 pmol/min/mm (p < 0.02 vs. NO; n = 5) (Fig. 2). We tested this concentration since those lower did not affect JCl [1]. Next, we evaluated the effect of 8-iso-PGF2α present in the basolateral side. Basal JCl was 235 ± 38 pmol/min/mm, which decreased in the presence of SNP (1 mM) to 139.4 ± 27 pmol/min/mm and increased to 297 ± 29 pmol/min/mm in the presence of 8-iso-PGF2α (100 μM; p < 0.02 vs. NO; n = 5) (Fig. 3). These data indicate that the maximal NO effect on cTAL is insufficient to prevent the 8-iso-PGF2α-stimulated NaCl reabsorption.
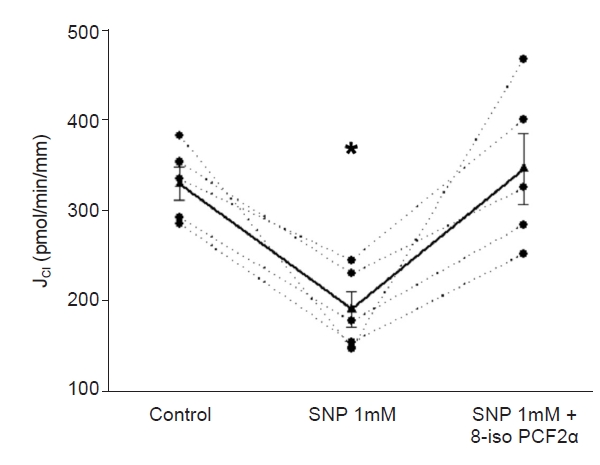
SNP-induced inhibition of JCl was prevented by 8-iso-PGF2α on the luminal side.
*p < 0.003 vs. basal; n = 5, one-way analysis of variance (Holm-Sidak test).
JCl, chloride absorption; SNP, sodium nitroprusside; 8-iso-PGF2α, 8-iso-prostaglandin-F2α.
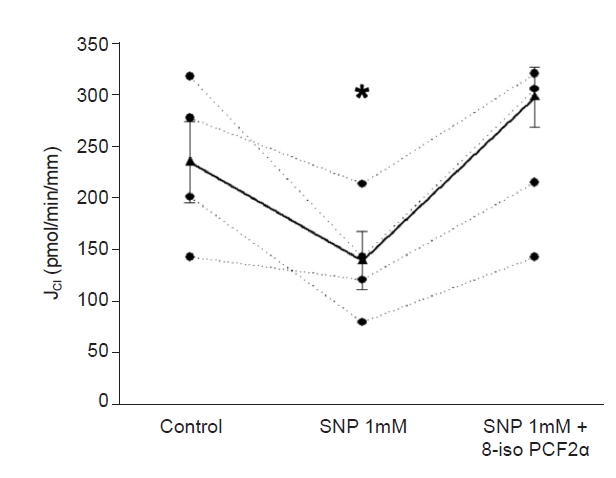
SNP-induced inhibition of JCl was prevented by 8-isoPGF2α on the basolateral side.
*p < 0.03 vs. control period; n = 4, one-way analysis of variance (Holm-Sidak test).
JCl, chloride absorption; SNP, sodium nitroprusside; 8-iso-PGF2α, 8-iso-prostaglandin-F2α.
In cTAL, 8-iso-PGF2α stimulates JCl via a cAMP-dependent mechanism [1], while NO inhibits chloride reabsorption by stimulation of a cGMP-stimulated phosphodiesterase, which decreases cAMP level [12]. Thus, we evaluated the effect of 8-iso-PGF2α on cAMP in the presence of a NO donor, SNP (1 mM). Basal cAMP was 56.3 ± 13.1 fmol/min/mm, while that in the presence of the NO donor was 57.8 ± 6.1 fmol/min/mm, the addition of 8-iso-PGF2α to which increased cAMP to 92.1 ± 2.9 fmol/min/mm (n = 10, p < 0.04) (Fig. 4). Thus, 8-iso-PGF2α-stimulated NO-inhibited JCl is associated with a 60% increase in cAMP.
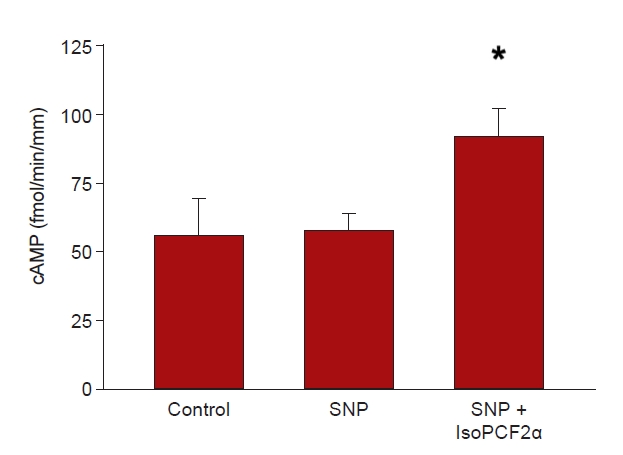
Effect of 100 μM 8-isoPGF2α on the presence of SNP, a NO donor.
Basal cAMP was 56.3 ± 13.1 fmol/min/mm, in the presence of the NO donor, 57.8 ± 6.1 fmol/min/mm, and 8-iso-PGF2α increased it to 92.1 ± 2.9 fmol/min/mm (*p < 0.001 vs. control and vs. SNP period; n = 10, one-way analysis of variance [Tukey honestly significant difference post hoc test]).
cAMP, cyclic adenosine monophosphate; JCl, chloride absorption; NO, nitric oxide; SNP, sodium nitroprusside; 8-iso-PGF2α, 8-iso-prostaglandin-F2α.
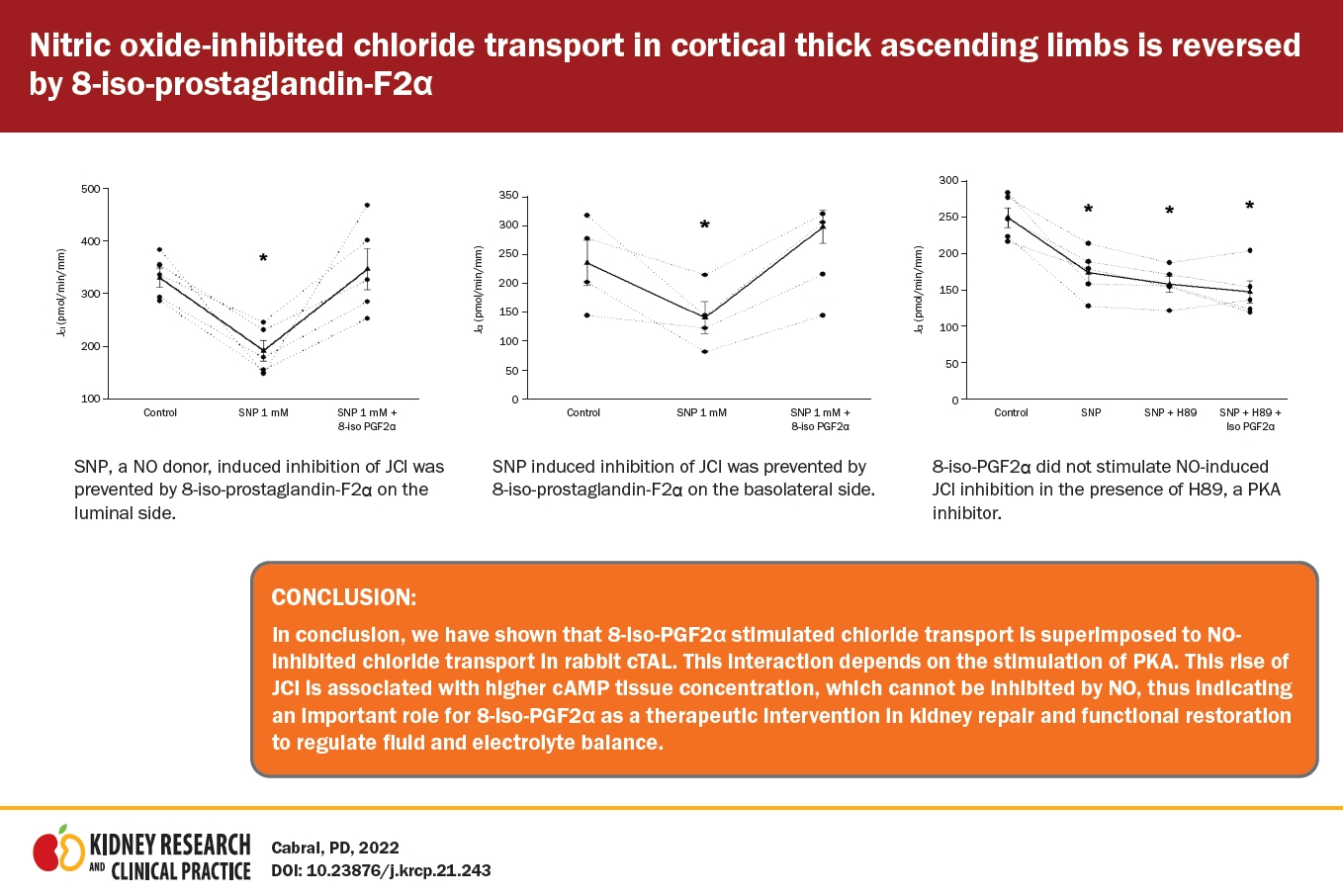
In the cTAL, PKA mediates the effect of 8-iso-PGF2α on JCl. Thus, we evaluated the effect of 8-iso-PGF2α on SNP-inhibited JCl in the presence of H89 (10 mM), a PKA inhibitor. Basal JCl was 249 ± 13 pmol/min/mm and decreased to 173 ± 14 pmol/min/mm when SNP (1 mM) was added to the bath. Addition of H89 to the bath did not change JCl significantly (158 ± 10 pmol/min/mm). Under these conditions, 8-iso-PGF2α could not stimulate SNP-inhibited JCl (147 ± 15 pmol/min/mm) (Fig. 5). Time control did not change during the experiment (280 ± 22 pmol/min/mm vs. 282 ± 36 pmol/min/mm). These data indicate that 8-iso-PGF2α requires PKA activity to reverse the NO-inhibited JCl.
Discussion
To our knowledge, this is the first report showing that 8-iso-PGF2α can override NO’s diuretic effects in the cTAL, suggesting that Na+ retention will prevail over Na+ excretion in clinical conditions associated with increased 8-iso-PGF2α level. Although in the kidney, discrete 8-iso-PGF2α and NO effects have both been shown in vivo and in vitro, we designed this in vitro investigation to identify the local interaction and final effect of these two autacoids on chloride transport at the nephron segment.
Despite the well-known importance of prostaglandin in Na reabsorption, the effects of its derivatives are less clear. We evaluated the effects of 8-iso-PGF2α binding to the G-protein coupled receptor thromboxane A2 (TXA2) receptor, localized in the thick ascending limb segment [13,14]. This 8-iso-PGF2α is a modified prostaglandin produced by nonenzymatic oxidation of prostaglandin-F2α by reactive oxygen species [15,16] and induces vasoconstriction in the kidney and can impact renal blood flow [17]. Our data demonstrate that the NO donor decreases chloride reabsorption in the thick ascending limb of Henle; under these circumstances, 8-iso-PGF2α is activated by PKA and stimulates Cl– reabsorption. In vivo, this interaction results in a decrease of natriuresis and sodium increase. The significance of this interaction resides not only in improving understanding of the normal physiology, but also in the prevention and treatment of many conditions such as hypertension, chronic renal failure, and cirrhosis, where isoprostanes increase and an Na retention state prevails [18].
The compound 8-iso-PGF2α binds the TXA2 receptor, a peroxidized derivative of prostaglandin-F2 localized in the basolateral and apical membrane of the thick ascending limb segment, which then activates cAMP production [14]. Following the release of 8-iso-PGF2α, sudden activation of the TXA2 receptor in the thick ascending limb occurs [1], stimulating chloride reabsorption via cAMP. Simultaneously, NO inhibits 8-iso-PGF2α via cGMP generation. Therefore, the interaction between these two intracellular mechanisms could regulate sodium transport.
In the kidney, 8-iso-PGF2α induces vasoconstriction, which can modulate renal blood flow [19]. In the thick ascending limb, TXA2 receptor release stimulates PKA and induces an increase in chloride reabsorption across the Na-K-Cl cotransporter 2 (NKCC2) [20]. Increased concentrations of 8-iso-PGF2α and other isoprostanes have been observed in both the urine and plasma of hypertensive subjects. The enhanced NaCl retention in response to 8-iso-PGF2α might contribute to the pathogenesis of hypertension [20]. In the distal nephron, NO decreases cAMP, which reduces fluid absorption via PKA [21].
We further investigated whether 8-iso-PGF2α could reverse NO effects when PKA is inhibited. This experiment should establish whether the interaction between the mechanisms linking 8-iso-PGF2α and NO precedes or follows activation of PKA. In the presence of PKA inhibition, 8-iso-PGF2α did not change JCl, indicating that PKA activity is required to reverse the NO-inhibited JCl. Moreover, these results indicate that 8-iso-PGF2α reverses NO-induced inhibition of JCl via a mechanism involving activation of PKA. Since PKA is mainly activated by cAMP, we studied cAMP and found that NO was not able to reduce the cAMP level induced by 8-iso-PGF2α.
Nevertheless, the effects of 8-iso-PGF2α and NO on NaCl excretion have been shown in several models [22]. The decreased urinary Na+ excretion caused by systemic nitric oxide synthase (NOS) inhibition correlates with higher urinary 8-iso-PGF2α [23,24]. Also, the superoxide scavenger Tempol blunts these changes, suggesting that inhibition of NO synthesis enhances endogenous superoxides, which could increase renal 8-iso-PGF2α level. These studies, however, show correlation but no direct interaction in the transport mechanisms. At any rate, 8-iso-PGF2α increases Cl– by stimulating cAMP synthesis [1].
In contrast, the effect by which NO inhibits NKCC2 activity might involve both an increase in cGMP [12,25,26] and a decrease in cAMP [27]. In our study, the NO donor did not decrease cAMP, whereas cGMP can play an essential role in the NO-inhibited JCl. This effect might involve protein trafficking, limiting the total pool of NKCC2 at the membrane level [28] since 8-iso-PGF2α’s increase of JCl is mediated by NKCC2 [1].
The mechanism(s) by which cGMP acts to regulate trafficking of the transporter might involve a decrease in insertion of the transporter into the membrane. Although evidence exists supporting the constitutive degradation of surface NKCC2, which is hypothesized to occur by decreasing NKCC2 level due to increasing NKCC2 ubiquitination and proteasomal degradation [28], it appears plausible that NO acts by increasing cGMP, which decreases cAMP [27] to lead to a reduction in exocytic insertion of NKCC2 into the apical membrane, reducing the number of transporters [19]. This process results in lower NKCC2 activity and blunted net NaCl reabsorption.
Our experiments demonstrate that interaction with 8-iso-PGF2α increases intracellular cAMP; in the presence of H89, 8-iso-PGF2α does not increase chloride reabsorption. These results show that the interaction occurs after activation of PKA since H89 prevented 8-iso-PGF2α and JCl inhibited NO and not because 8-iso-PGF2α blunts cGMP formation. Indeed, we found that NO caused no significant change in 8-iso-PGF2α-stimulated cAMP level. This indicates that NO-stimulated phosphodiesterase activity has little or no effect on 8-iso-PGF2α-stimulated cAMP level. This notion might not apply to other cellular compartments, where NO-stimulated phosphodiesterase could be activated separately from 8-iso-PGF2α-stimulated cAMP. Thus, 8-iso-PGF2α might interact with NO at the level of cAMP or cGMP.
The pathomechanism whereby 8-iso-PGF2α prevents NO-inhibited JCl might be observed under several conditions characterized by increased blood pressure, such as hypertension and chronic renal failure. In such conditions, urinary 8-iso-PGF2α 9 [29] and cAMP [30] are elevated as well as other oxidative stress mediators. On the contrary, NO production is impaired in part due to limitations on substrate (L-arginine) availability and increased circulating levels of endogenous NO synthase inhibitors, in particular asymmetric dimethylarginine and reduced renal cortex abundance of the neuronal NOS [31]. This effect of NO correlates with urinary cGMP level [32]. This pathological condition is characterized by sodium retention as a consequence of enhanced sympathetic activity [33] and increase synthesis of intrarenal angiotensin II synthesis [34], endothelin-1 [35], and 8-iso-PGF2α, all of which stimulate sodium reabsorption. In contrast, the antinatriuretic effect of NO is blunted under these conditions, leading to sodium retention, hypertension, fibrosis, and end-organ damage.
The beneficial effect of 8-iso-PGF2α blockade has been observed in several animal models. Studies in diabetic animals with nephropathy showed that supplementation with vitamin E via reduced production of transforming growth factor-beta reduced urinary isoprostane level and improved proteinuria and blood urea nitrogen level [36]. In spontaneously animal models of hypertension and angiotensin II-induced hypertension, both with elevated plasma isoprostane level, Tempol administration decreases renal isoprostane excretion and lowers blood pressure [4,37].
These beneficial effects observed by isoprostane blockade (and increase in NO availability) can prevent sodium overload, hypertension, chronic fibrosis, and end-organ damage. Clinical evaluations are under review to estimate the beneficial effect of isoprostane blockade (NCT03358524, NCT01125501, and NCT00552227).
Although the results of this report can explain important pathophysiological conditions, it has some limitations. One important limitation is the H89, despite being the most commonly used PKA inhibitor, might have nonspecific inhibitory effects.
In conclusion, 8-iso-PGF2α can increase JCl at the kidney cTAL via stimulation of PKA even when JCl is inhibited by NO. This increase of JCl is associated with a higher cAMP tissue concentration, which cannot be inhibited by NO, indicating the importance of 8-iso-PGF2α as a therapeutic intervention in kidney repair and functional restoration to regulate fluid and electrolyte balance.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This work was partially supported by Grant PIP 6525 from CONICET, Argentina, and by grant from the J. Robert Cade Foundation (No. L#003).
Authors’ contributions
Conceptualization, Funding acquisition: LIJ, NHG
Investigation, Investigation, Methodology: PDC, STB, GBS
Data curation, Formal analysis: GBS, LIJ, NHG
Writing–original draft: GBS, NHG
Writing–review & editing: PDC, STB, LIJ, EIOA, NHG
All authors read and approved the final manuscript.
Acknowledgements
Néstor H. García acknowledges the support from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).