1. Muto S, Furuse M, Kusano E. Claudins and renal salt transport.
Clin Exp Nephrol 2012;16:61–67.


2. Li J, Ananthapanyasut W, Yu AS. Claudins in renal physiology and disease.
Pediatr Nephrol 2011;26:2133–2142.



3. Lee DB, Huang E, Ward HJ. Tight junction biology and kidney dysfunction.
Am J Physiol Renal Physiol 2006;290:F20–F34.


4. Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions.
J Cell Biol 1993;123(6 Pt 2):1777–1788.



5. Gonzalez-Mariscal L, Namorado MC, Martin D, et al. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules.
Kidney Int 2000;57:2386–2402.


6. Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands.
Mol Biol Cell 2000;11:4131–4142.



7. Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin.
J Cell Biol 1998;141:1539–1550.



8. Tsukita S, Tanaka H, Tamura A. The claudins: from tight junctions to biological systems.
Trends Biochem Sci 2019;44:141–152.


9. Tamura A, Tsukita S. Paracellular barrier and channel functions of TJ claudins in organizing biological systems: advances in the field of barriology revealed in knockout mice.
Semin Cell Dev Biol 2014;36:177–185.


10. Hou J, Rajagopal M, Yu AS. Claudins and the kidney.
Annu Rev Physiol 2013;75:479–501.


11. Bhat AA, Syed N, Therachiyil L, et al. Claudin-1, a double-edged sword in cancer.
Int J Mol Sci 2020;21:569.



12. Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells.
J Cell Biol 2001;153:263–272.



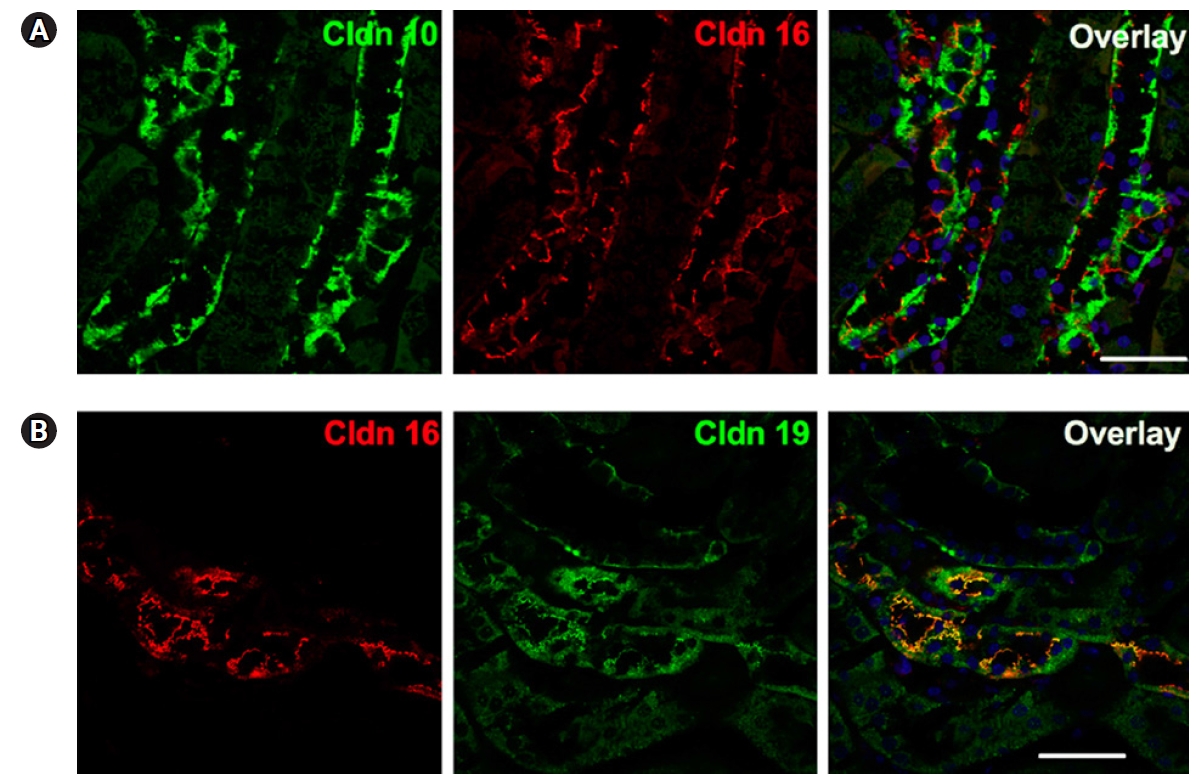
13. Milatz S, Himmerkus N, Wulfmeyer VC, et al. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport.
Proc Natl Acad Sci U S A 2017;114:E219–E227.


14. Breiderhoff T, Himmerkus N, Stuiver M, et al. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis.
Proc Natl Acad Sci U S A 2012;109:14241–14246.



15. Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities.
Am J Physiol Renal Physiol 2006;291:F1288–F1299.


16. Günzel D, Stuiver M, Kausalya PJ, et al. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function.
J Cell Sci 2009;122(Pt 10):1507–1517.


17. Plain A, Pan W, O’Neill D, et al. Claudin-12 knockout mice demonstrate reduced proximal tubule calcium permeability.
Int J Mol Sci 2020;21:2074.



18. Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions.
J Cell Sci 2005;118(Pt 21):5109–5118.


19. Kausalya PJ, Amasheh S, Günzel D, et al. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16.
J Clin Invest 2006;116:878–891.



20. Ikari A, Hirai N, Shiroma M, et al. Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells.
J Biol Chem 2004;279:54826–5432.


21. Ikari A, Matsumoto S, Harada H, et al. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions.
J Cell Sci 2006;119(Pt 9):1781–1789.


22. Hou J, Renigunta A, Gomes AS, et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium.
Proc Natl Acad Sci U S A 2009;106:15350–15355.



23. Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization.
Proc Natl Acad Sci U S A 2010;107:18010–18015.



24. Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference.
J Biol Chem 2006;281:36117–36123.


25. Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability.
J Clin Invest 2001;107:1319–1327.



26. Krug SM, Günzel D, Conrad MP, et al. Claudin-17 forms tight junction channels with distinct anion selectivity.
Cell Mol Life Sci 2012;69:2765–2778.


27. Conrad MP, Piontek J, Günzel D, Fromm M, Krug SM. Molecular basis of claudin-17 anion selectivity.
Cell Mol Life Sci 2016;73:185–200.


28. Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5.
Mol Cell Biol 2004;24:8408–8417.



29. Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation.
J Biol Chem 2003;278:17350–17359.


30. Angelow S, Kim KJ, Yu AS. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells.
J Physiol 2006;571(Pt 1):15–26.


31. Ben-Yosef T, Belyantseva IA, Saunders TL, et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration.
Hum Mol Genet 2003;12:2049–2061.


32. Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT. Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion.
Am J Physiol Renal Physiol 2013;304:F761–F769.


33. Jovov B, Van Itallie CM, Shaheen NJ, et al. Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance.
Am J Physiol Gastrointest Liver Physiol 2007;293:G1106–G1113.


34. Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal localization and function of the tight junction protein, claudin-19.
Am J Physiol Renal Physiol 2007;293:F166–F177.


35. Sas D, Hu M, Moe OW, Baum M. Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells.
Am J Physiol Regul Integr Comp Physiol 2008;295:R1713–R1719.



36. Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl- permeability.
Biochem Biophys Res Commun 2007;357:87–91.


37. Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells.
J Cell Sci 2005;118(Pt 12):2683–2693.


38. Hou J, Renigunta A, Konrad M, et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex.
J Clin Invest 2008;118:619–628.



39. Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells.
Eur J Cell Biol 1999;78:849–855.


40. McCarthy KM, Francis SA, McCormack JM, et al. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells.
J Cell Sci 2000;113 Pt 19:3387–3398.


41. Milatz S, Krug SM, Rosenthal R, et al. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes.
Biochim Biophys Acta 2010;1798:2048–2057.


42. Rosenthal R, Milatz S, Krug SM, et al. Claudin-2, a component of the tight junction, forms a paracellular water channel.
J Cell Sci 2010;123(Pt 11):1913–1921.


43. Gong Y, Sunq A, Roth RA, Hou J. Inducible expression of claudin-1 in glomerular podocytes generates aberrant tight junctions and proteinuria through slit diaphragm destabilization.
J Am Soc Nephrol 2017;28:106–117.


44. Kong L, Wu H, Zhou W, et al. Sirtuin 1: a target for kidney diseases.
Mol Med 2015;21:87–97.



45. Hasegawa K, Wakino S, Simic P, et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes.
Nat Med 2013;19:1496–1504.



46. Koda R, Zhao L, Yaoita E, et al. Novel expression of claudin-5 in glomerular podocytes.
Cell Tissue Res 2011;343:637–648.


47. Zhao L, Yaoita E, Nameta M, et al. Claudin-6 localized in tight junctions of rat podocytes.
Am J Physiol Regul Integr Comp Physiol 2008;294:R1856–R1862.


48. Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM. Tight junctions of the proximal tubule and their channel proteins.
Pflugers Arch 2017;469:877–887.


49. Muto S, Hata M, Taniguchi J, et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules.
Proc Natl Acad Sci U S A 2010;107:8011–8016.



50. Yu AS. Paracellular transport and energy utilization in the renal tubule.
Curr Opin Nephrol Hypertens 2017;26:398–404.


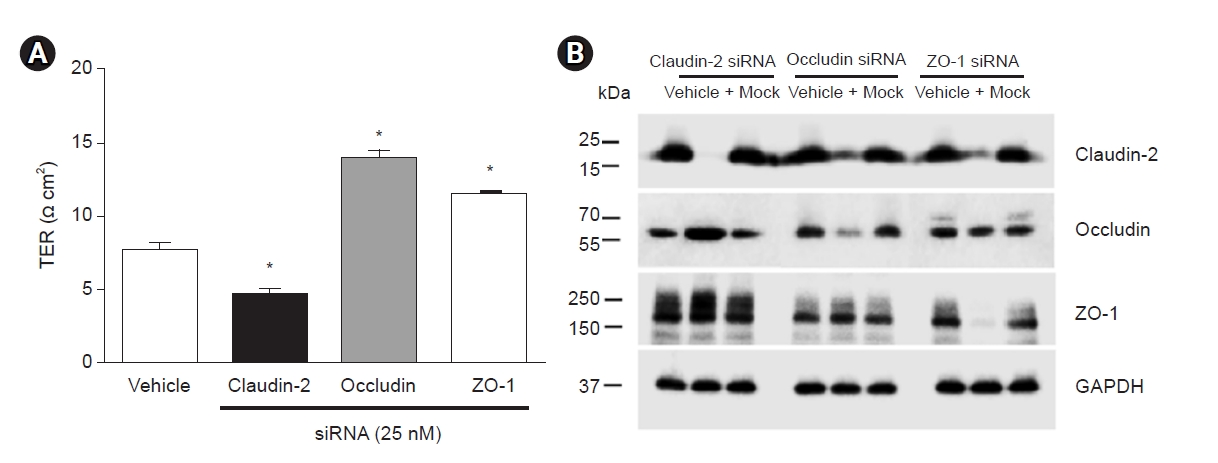
51. Kim S, Kim GH. Roles of claudin-2, ZO-1 and occludin in leaky HK-2 cells.
PLoS One 2017;12:e0189221.



52. Curry JN, Yu AS. Paracellular calcium transport in the proximal tubule and the formation of kidney stones.
Am J Physiol Renal Physiol 2019;316:F966–F969.



53. Curry JN, Tokuda S, McAnulty P, Yu AS. Combinatorial expression of claudins in the proximal renal tubule and its functional consequences.
Am J Physiol Renal Physiol 2020;318:F1138–F1146.



54. Pei L, Solis G, Nguyen MT, et al. Paracellular epithelial sodium transport maximizes energy efficiency in the kidney.
J Clin Invest 2016;126:2509–2518.



55. Tanaka H, Tamura A, Suzuki K, Tsukita S. Site-specific distribution of claudin-based paracellular channels with roles in biological fluid flow and metabolism.
Ann N Y Acad Sci 2017;1405:44–52.


56. Curry JN, Saurette M, Askari M, et al. Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease.
J Clin Invest 2020;130:1948–1960.



57. Balkovetz DF, Chumley P, Amlal H. Downregulation of claudin-2 expression in renal epithelial cells by metabolic acidosis.
Am J Physiol Renal Physiol 2009;297:F604–F611.


58. Olinger E, Houillier P, Devuyst O. Claudins: a tale of interactions in the thick ascending limb.
Kidney Int 2018;93:535–537.


59. Plain A, Alexander RT. Claudins and nephrolithiasis.
Curr Opin Nephrol Hypertens 2018;27:268–276.


60. Prot-Bertoye C, Houillier P. Claudins in renal physiology and pathology.
Genes (Basel) 2020;11:290.



61. Moor MB, Bonny O. Ways of calcium reabsorption in the kidney.
Am J Physiol Renal Physiol 2016;310:F1337–F1350.


62. Hou J. Claudins and mineral metabolism.
Curr Opin Nephrol Hypertens 2016;25:308–313.



63. Gong Y, Hou J. Claudin-14 underlies Ca⁺⁺-sensing receptor-mediated Ca⁺⁺ metabolism via NFAT-microRNA-based mechanisms.
J Am Soc Nephrol 2014;25:745–760.


64. Oh IH, Jo CH, Kim S, Jo S, Chung S, Kim GH. Thick ascending limb claudins are altered to increase calciuria and magnesiuria in metabolic acidosis.
Am J Physiol Renal Physiol 2021;320:F418–F428.


65. Gong Y, Renigunta V, Himmerkus N, et al. Claudin-14 regulates renal Ca⁺⁺ transport in response to CaSR signalling via a novel microRNA pathway.
EMBO J 2012;31:1999–2012.



66. Arcidiacono T, Simonini M, Lanzani C, et al. Claudin-14 gene polymorphisms and urine calcium excretion.
Clin J Am Soc Nephrol 2018;13:1542–1549.



67. Meyers N, Nelson-Williams C, Malaga-Dieguez L, et al. Hypokalemia associated with a claudin 10 mutation: a case report.
Am J Kidney Dis 2019;73:425–428.


68. Leiz J, Schmidt-Ott KM. Claudins in the renal collecting duct.
Int J Mol Sci 2019;21:221.



69. Gong Y, Yu M, Yang J, et al. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation.
Proc Natl Acad Sci U S A 2014;111:E3766–E3774.



70. Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8.
Proc Natl Acad Sci U S A 2015;112:4340–4345.



71. Fan J, Tatum R, Hoggard J, Chen YH. Claudin-7 modulates Cl- and Na+ homeostasis and WNK4 expression in renal collecting duct cells.
Int J Mol Sci 2019;20:3798.



72. Tatum R, Zhang Y, Salleng K, et al. Renal salt wasting and chronic dehydration in claudin-7-deficient mice.
Am J Physiol Renal Physiol 2010;298:F24–F34.


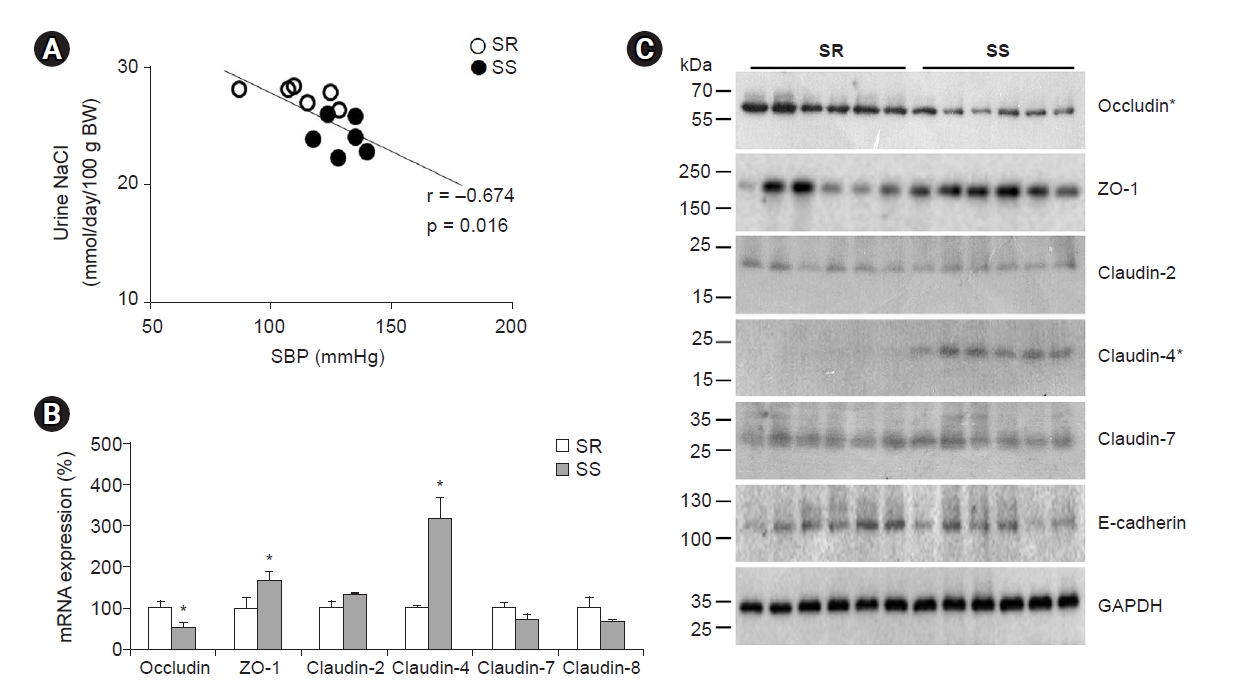
73. Jo CH, Kim S, Oh IH, Park JS, Kim GH. Alteration of tight junction protein expression in Dahl salt-sensitive rat kidney.
Kidney Blood Press Res 2017;42:951–960.


74. Stahl RA, Kanz L, Maier B, Schollmeyer P. Hyperchloremic metabolic acidosis with high serum potassium in renal transplant recipients: a cyclosporine A associated side effect.
Clin Nephrol 1986;25:245–248.

75. Lee CH, Kim S, Kang CM, Kim WY, Kim J, Kim GH. Altered expression of tight junction proteins in cyclosporine nephrotoxicity.
Am J Nephrol 2011;33:7–16.


76. Sassi A, Wang Y, Chassot A, et al. Interaction between epithelial sodium channel γ-subunit and claudin-8 modulates paracellular sodium permeability in renal collecting duct.
J Am Soc Nephrol 2020;31:1009–1023.



77. Bleich M, Günzel D. Physiology, pathophysiology, and clinical impact of claudins.
Pflugers Arch 2017;469:1–2.










 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















