Introduction
Acute kidney injury (AKI) occurs in 14% to 46% of patients with rhabdomyolysis [
1]. When muscle cells are damaged, intracellular myoglobin is released into the circulation causing renal damage due to direct tubule injury, tubular obstruction, or renal vasoconstriction. Subsequently, a large amount of water is rapidly expelled into the extracellular fluid, causing a decreased glomerular filtration rate with worsening of AKI [
2]. Life-threatening AKI leads to electrolyte and volume imbalances, which can cause arrhythmia or cardiac arrest, and renal replacement therapy is required. de Meijer et al. [
3] reported that mortality in patients who developed AKI was twice as high as in those who did not. Therefore, for patients with severe rhabdomyolysis with a high risk of AKI and death, judicious and timely treatment remains essential.
The hypotheses that acidic urine worsens acute tubulonephropathy and that the risk of AKI is associated with dehydration were used to support bicarbonate therapy for patients with rhabdomyolysis, wherein bicarbonate inhibits myoglobin cast formation, and a large amount of fluid results in solute diuresis due to alkalization of the urine [
4]. However, there are only a few studies, with no clear consensus, on whether the routine use of bicarbonate can prevent the development of AKI [
5,
6]. No randomized controlled clinical trials have compared bicarbonate therapy with fluid therapy alone. Indeed, some studies suggested that early, rather than late initiation of fluid therapy can help improve outcomes [
7–
9]; however, fluid type and target fluid volume, duration of therapy, monitoring parameters, target urine output, and the onset of initiation of fluid therapy used in these studies varied widely. Various studies have attempted to identify the optimal fluid therapy in various diseases or situations, such as renal transplant [
10]. However, no prior study has investigated what fluid is optimal for the treatment of rhabdomyolysis despite the importance of fluid therapy in this condition. Thus, we investigated whether bicarbonate therapy could prevent AKI compared to fluid therapy alone and assessed the effect of fluid volume on patient outcomes. Further, we analyzed predictors of AKI, dialysis, and death in patients hospitalized for rhabdomyolysis.
Discussion
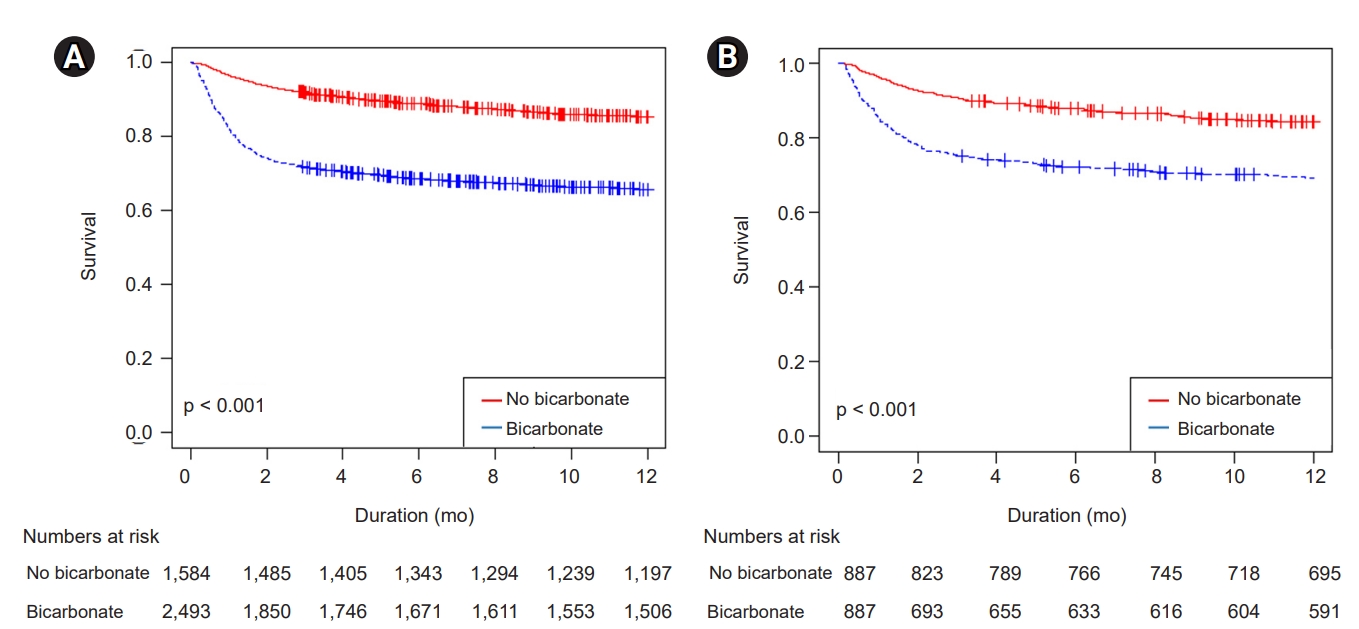
In this retrospective, propensity score-matched cohort study involving patients with rhabdomyolysis, we concluded that bicarbonate therapy increased AKI risk, the need for dialysis, and mortality. Additional hypervolemic treatment also appeared to be deleterious as it was associated with volume overload and resulted in poor renal outcomes.
Early fluid resuscitation and bicarbonate therapy had been advocated since a 1984 study of seven participants with crush injuries reported treatment by the induction of alkaline solute diuresis [
13]; since then, however, results concerning the outcomes of bicarbonate treatment for the prevention of AKI have been conflicting [
5,
14–
17]. In a previous retrospective study of more than 2,000 patients with traumatic rhabdomyolysis, bicarbonate with mannitol did not prevent AKI, the need for dialysis, or decrease mortality. Thus, the authors concluded that the use of bicarbonate with mannitol should be reevaluated [
6]. Several meta-analyses have noted the lack of prospective controlled trials comparing bicarbonate therapy with fluid therapy alone for preventing rhabdomyolysis-induced AKI [
8,
9].
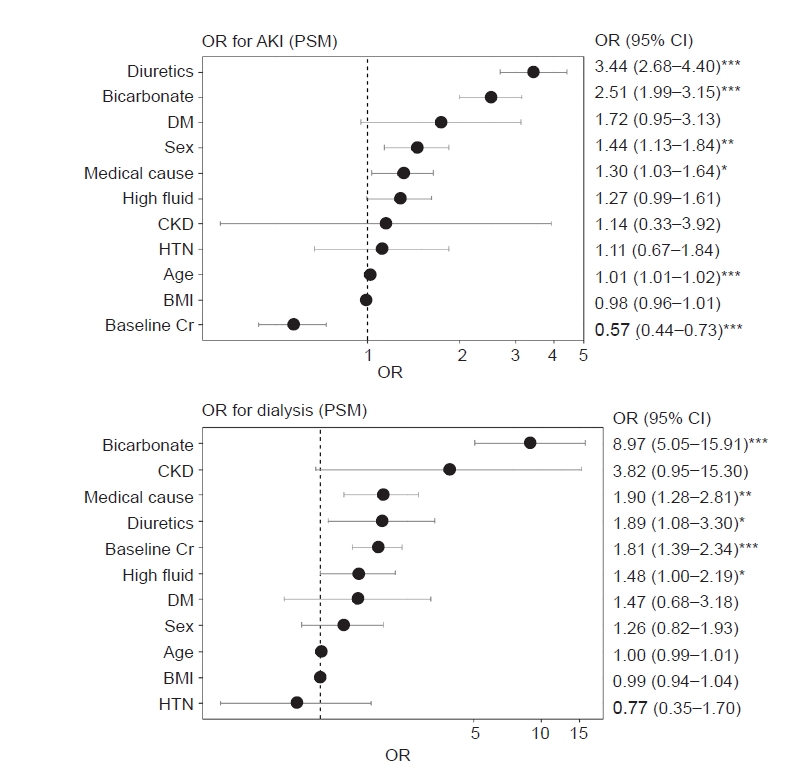
In our study, higher rates of AKI in the bicarbonate group might have resulted from several factors. First, clinicians may have used bicarbonate therapy
a priori in patients with presumptive high-risk factors with worse expected disease courses because its use was at the discretion of the treating physicians. We used propensity score matching by adjusting for underlying disease, sex, volume and BP status, baseline Cr level, and baseline CK level in an attempt to correct for this; however, the bicarbonate group still presented with a higher AKI incidence, need for dialysis, and mortality. Based on a review of the literature for other studies with higher AKI rates in the bicarbonate group than in the non-bicarbonate group, we found one study that reported that of 157 patients, 16.5% had AKI and the incidence of AKI was significantly higher in those patients who received bicarbonate therapy; however, the authors speculated that bicarbonate administration was not the cause of AKI but the result of receiving more aggressive therapy [
18]. However, this study was not designed to identify the difference between bicarbonate and non-bicarbonate groups; thus, causal relations could not be assessed. Second, bicarbonate therapy may potentially have detrimental effects. The effects of volume and sodium overload are not disputable [
19]. Consequences of bicarbonate therapy are hypervolemia, hyperosmolarity, and hypernatremia. Bicarbonate infusion may induce hyperlactatemia. In contrast to the beneficial use of bicarbonate for bicarbonate-losing metabolic acidosis, such as metabolic acidosis caused by diarrhea or renal tubular acidosis, the use of bicarbonate for lactic acidosis has been disproved [
19]. Furthermore, a hyperosmolar state caused by bicarbonate infusion may lead to paradoxical intracellular acidosis [
8,
20]. Additionally, serum ionized calcium level is reduced by bicarbonate infusion, and hypocalcemia is associated with reduced left ventricular contractility [
21]. These reactions may offset any beneficial effect of alkalization of urine, and bicarbonate therapy may contribute to the development of AKI. We found that hypernatremia, volume overload, and other electrolyte imbalances were more prominent in the bicarbonate group than in the non-bicarbonate group, although the rates of hypocalcemia were not significantly different between the bicarbonate and non-bicarbonate groups, and lactate levels were not measured.
Hypervolemic treatment may also contribute to the development of AKI [
2]. The primary mechanism of rhabdomyolysis-induced AKI is renal vasoconstriction; thus, early intravenous fluid treatment has been used to restore blood flow and glomerular filtration to protect the kidneys. Surprisingly, previous studies have shown that a greater degree of kidney dysfunction results from venous congestion transmitted to the renal venous compartment than from lack of arterial perfusion [
22,
23]. Therefore, aggressive volume treatment to maintain renal flow may contribute to peripheral overload, resulting in renal congestion and AKI. We demonstrated that patients with volume overload were at increased risk of developing AKI. Moreover, volume overload was an independent risk factor for mortality in our study. Our results are consistent with those of a previous study that reported that volume overload increases the risk of AKI and mortality, independent of the severity of acute illness, indicating that prevention of volume overload is crucial in managing critically ill patients [
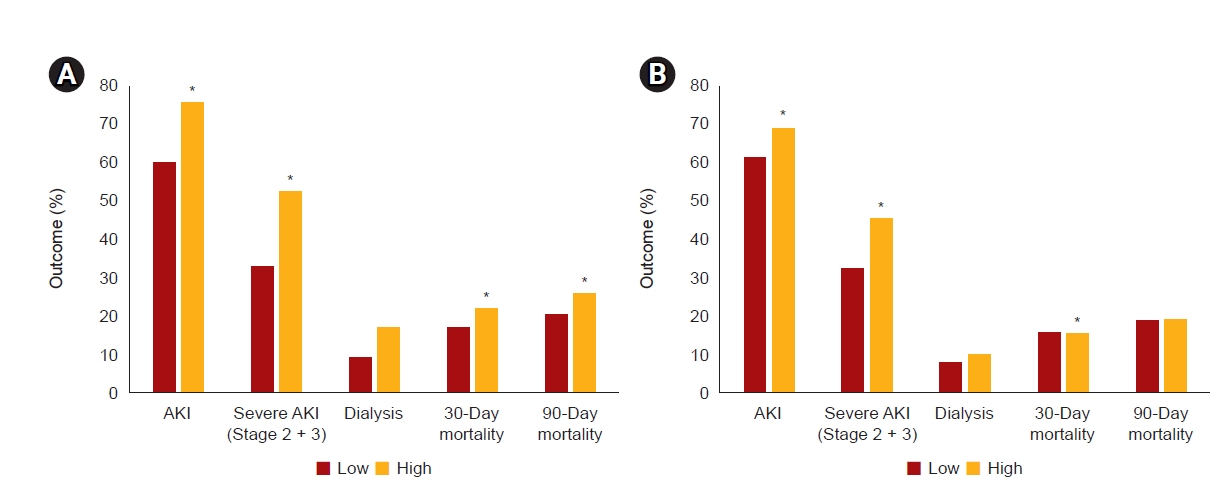
24]. We stratified high-volume fluid therapy and bicarbonate therapy using RERI analysis, and each variable independently and synergistically had detrimental effects on the development of AKI. We suggest that <5.5 mL/kg/hr fluid resuscitation should be used to prevent AKI and mortality in patients with rhabdomyolysis, and volume overload, hourly urine output, and central venous pressure should be monitored periodically. Nevertheless, bicarbonate use may have a role in severe acidemic patients with rhabdomyolysis. Despite the lack of relevant clinical data supporting its effectiveness, theoretical evidence still exists [
25–
27].
Although the risk of AKI was higher for medically-caused rhabdomyolysis than surgically-induced rhabdomyolysis, the association between bicarbonate use and AKI was higher in patients with rhabdomyolysis with a surgical cause. In terms of AKI, bicarbonate use appeared harmful in patients who were postsurgical, hypothermic, or immobilized, whereas bicarbonate use appeared beneficial in cases of crush, seizure, hyperthermia, and malignant neuroleptic syndrome. However, given the large numerical differences in causal subcategories in our cohort, further subanalyses are required to elucidate important subgroup effects. Therefore, we propose a large-scale cohort study wherein patients have rhabdomyolysis of homogeneous etiology.
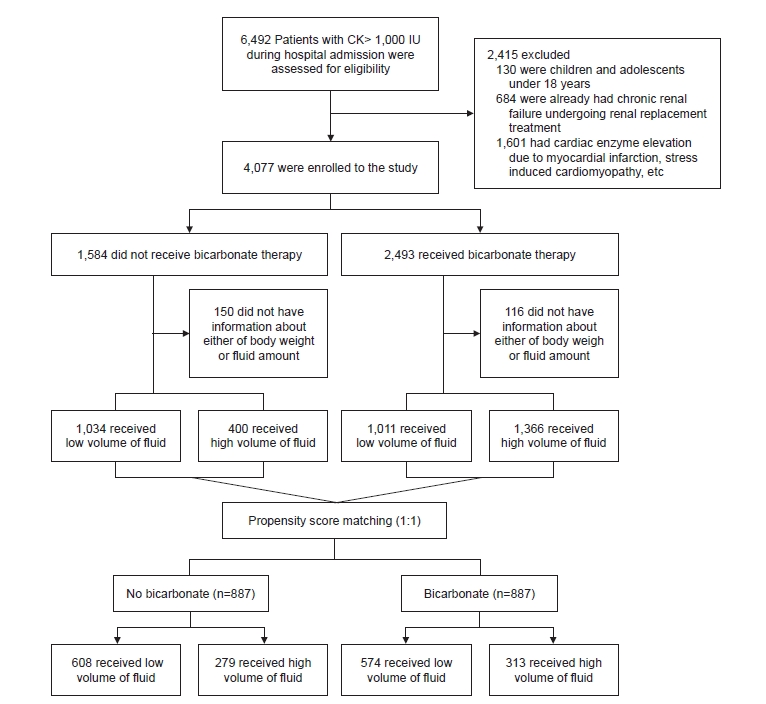
Our study had several strengths. We used propensity score-matched analyses to reduce selection bias and potential baseline differences between bicarbonate and non-bicarbonate groups. AKI was defined and staged according to standard definitions proposed by the KDIGO Group [
11]; therefore, the study results are likely generalizable to patients at other institutions. More than 4,000 patients were enrolled, and after propensity score matching, over 3,000 patients were included in the analyses, which to the best of our knowledge is the largest cohort of rhabdomyolysis patients analyzed in a single study.
Our study also has several limitations. First, it was not specifically designed to illustrate the initiation and maintenance of bicarbonate therapy, bicarbonate dosage, or target serum and urine pH levels. We did not tailor the infusion of bicarbonate according to serum or urine pH levels. Second, information on the type of fluid was not stratified. Different fluid mixtures such as isotonic saline and dextrose fluids were used in our study in the real-world setting. However, the amount of fluid was adjusted by body weight to set individual target volumes. Third, although we separated medical and surgical causes of rhabdomyolysis, numerous heterogeneous causes of rhabdomyolysis were included. Fourth, although we performed propensity score matching to avoid potential bias in the use of bicarbonate in patients with predicted worse outcomes, such as severe acidemia, volume depletion, or state of shock, there may have been confounding effects due to complex and unintended treatment settings. Future research should prospectively evaluate the need for bicarbonate infusion and the amount of fluid therapy without these limitations.
In conclusion, we found that the use of bicarbonate and high-volume fluid treatment that resulted in volume overload were not beneficial but rather harmful to a certain subset of patients with rhabdomyolysis, as reflected by increased AKI risk, the need for dialysis, and increased mortality.
















 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















