| Kidney Res Clin Pract > Volume 35(4); 2016 > Article |
|
Abstract
Crystalline nephropathy is a rare yet well-known condition associated with multiple myeloma and other light chain–secreting disorders. Paraproteins that are resistant to proteolysis crystallize within proximal tubular cells and cause light-chain proximal tubulopathy, which presents clinically as Fanconi syndrome. Podocytes are rarely affected, and the crystalline inclusions within podocytes are typically precipitated, yielding significant glomerular proteinuria. Here we report a case of extensive crystalline inclusions primarily within podocytes and proximal tubules that presented only with Fanconi syndrome and renal insufficiency. Despite the presence of extensive crystalline inclusions in podocytes and diffuse foot process effacement, the patient had no clinical evidence suggestive of podocyte injury.
Keywords
Fanconi syndrome, Multiple myeloma, NephropathyRenal disease is common in multiple myeloma, with almost 50% of all patients showing renal dysfunction at initial presentation [1]. Intrarenal crystalline deposits of monoclonal immunoglobulins and/or light-chain molecules are an important cause of renal dysfunction [2]. Light-chain cast nephropathy is the most common form of crystalline nephropathy and is caused by large amounts of monoclonal light chains produced in multiple myeloma. Freely filtered light chains over the reabsorptive capacity of proximal tubules lead to light-chain proteinuria. Light chains precipitate in the tubules as a result of their binding to the Tamm–Horsfall mucoprotein. This binding leads to characteristic crystalline precipitates of monoclonal light chains within distal tubules [3]. Intracellular crystallization also occurs less commonly within proximal tubular cells, leading to light-chain proximal tubulopathy (LCPT). The proximal tubules are most prominently affected because of the reabsorption of filtered light chains and their subsequent crystal accumulation in the proximal tubules. As a result, patients with LCPT typically present with features of Fanconi syndrome [4].
In contrast to depositions in tubular epithelial cells, crystal depositions in glomerular cells are relatively uncommon. When podocytes are affected, the crystalline inclusions within the podocytes are typically associated with significant glomerular proteinuria [5].
Here we report a patient with multiple myeloma who presented with Fanconi syndrome and renal insufficiency. Renal biopsy showed characteristic features of LCPT, including crystalline inclusions within tubular epithelial cells. Moreover, ultrastructural examinations of the glomeruli showed extensive accumulation of intracellular crystalline inclusions within the podocytes and diffuse effacement of foot processes. Despite these histologic findings, the patient did not show overt glomerular proteinuria or glomerular hematuria.
A 66-year-old woman was admitted to our hospital for evaluation of her back pain. She had chronic hepatitis B and suffered from hepatic cell carcinoma. Lumbar magnetic resonance imaging revealed bone lesions suggesting metastasis. Surgical treatment was planned to relieve her pain. Her preoperative laboratory examination revealed renal insufficiency, and she was thus referred to the nephrology department. Her serum laboratory results were indicative of renal dysfunction (creatinine 1.7 mg/dL, blood urea nitrogen 22.3 mg/dL), hypouricemia (uric acid 1.7 mg/dL), hypophosphatemia (phosphate 2.4 mg/dL), and a reversed albumin/globulin ratio (albumin 2.9 g/dL, globulin 7.8 g/dL). The urine dipstick test showed a specific gravity of 1.010, blood (±), albumin (±), and glucose (+++). However, the spot urine test revealed proteinuria with a urine protein/creatinine ratio of 3.11 µg/mg and a urine albumin/creatinine ratio of 244.2 μg/mg. Abnormal bands were observed against anti-IgG and anti-κ light chain in serum and urine immunofixation analyses. Her serum-free κ/λ light-chain ratio was 246, and her level of serum M-protein was 5.04 g/dL. Her ß2-microglobulin was 7.3 mg/L. Urine protein electrophoresis showed 2 peaks; the levels of urine M-protein were 944.6 and 87.7 mg/d.
A kidney biopsy was performed to ensure accurate diagnosis of renal insufficiency and Fanconi syndrome. Examination by light microscopy identified 1 renal cortex core containing 49 glomeruli, 3 of which showed global sclerosis but no focal segmental glomerulosclerosis (FSGS). The glomeruli were generally unremarkable, with the exception of mild wrinkling of the capillary tufts (Fig. 1). The tubules were in different stages of damage and showed variable thickening of the basement membranes. They also occasionally contained intraluminal casts in the form of loose concretions that appeared pale pink by periodic acid-Schiff staining. In podocytes we could focally find small inclusion like structures. Immunofluorescence staining revealed scattered dot–like positivity for the anti-κ light chain but not for the λ analysis, which were present in overall cells showing positive reaction including glomeruli, tubules. Ultrastructural analysis revealed oval or angulated crystalline inclusions that were present in the cellular organelles, such as phagolysosomes, in many different types of cells or that were freely floating in the cytoplasm. Crystalline inclusions were almost confined in the podocytes (Fig. 2). Mesangial and endothelial cells were also affected. The tubular epithelial cells contained intracellular crystalline inclusions that were also found in the luminal space. Light chain crystals in the tubular lumen were also seen (Fig. 3). Some tubules contained intraluminal casts composed of degenerated crystalline material; crystalline inclusions similar to those found in the cytoplasm were present as foci along the periphery of the cast (Fig. 4). Outside the tubules, the interstitial cells were similarly laden with the crystalline structures. Crystalline inclusions were found in interstitial cells, presumably histiocytes.
Bone marrow biopsy showed 30% monoclonal plasma cell proliferation with κ restriction. Finally, she was diagnosed with multiple myeloma with Fanconi syndrome accompanied by extensive crystalline nephropathy. The first cycle of chemotherapy was given immediately after her diagnosis of multiple myeloma [Bortezomib (Velcade), melphalan, and prednisolone with dose reduction]. Disease evaluation after 1 cycle of chemotherapy revealed overall improvement in her myeloma-related laboratory results.
In this case, a 66-year-old woman presented with renal insufficiency and Fanconi syndrome. Her laboratory results showed a reversed albumin/globulin ratio and nonalbumin dominant proteinuria. She was diagnosed with IgG-κ multiple myeloma based on this evaluation. A kidney biopsy was performed to identify the cause of her acquired Fanconi syndrome and renal insufficiency. Her renal pathologic findings revealed extensive and generalized deposition of crystals in podocytes, mesangial cells, interstitial histiocytes, and the intraluminal cast, in addition to intracytoplasmic crystalline depositions in the proximal tubular epithelium. In addition, despite the massive infiltration of crystals into podocytes with foot process effacement, the patient did not show features of nephrotic syndrome.
Crystalline nephropathy is a very rare disease associated with paraproteinemia. Depending on the crystal distribution, paraprotein-induced crystalline nephropathy can be divided into 2 categories, intracellular and extracellular [6].
In our case, the most striking pathologic finding was extensive and generalized deposition of crystals observed simultaneously in both the intracellular and extracellular spaces. However, the prominent infiltration of crystals into podocytes without nephrotic features is inconsistent with previous case reports [5]. Crystalline podocytopathy is extremely rare, with only 11 cases reported in literature to the best of our knowledge. These cases showed features of significant podocyte injury including nephrotic or subnephrotic glomerular proteinuria and FSGS on renal biopsy [7]. The reported cases showed diverse locations of crystal deposition such as podocytes, tubular epithelial cells, mesangial cells, interstitial histiocytes which were similar with our case. There was no FSGS in this case, despite sclerotic glomeruli implying FSGS was common findings in other reports [8]. Interestingly, in this case, despite the crystal deposition in the podocytes and the foot process effacement, the patient did not show significant glomerular proteinuria (urine albumin/creatinine ratio of 244.2 μg/mgCr) at the time of biopsy. Although the reasons underlying the absence of overt glomerular proteinuria are presently unknown, we speculate that two possibilities. Three cases described nephrotic syndrome accompanying with FSGS and two cases showed collapsing glomerulopathy which resulted from catastrophic injury to podocytes [5], [9], [10]. No FSGS in pathologic findings and no risk factors to collapsing glomerulopathy in this case were related with subnephrotic proteinuria. Also, we supposed that nephrotic features might eventually occur if the diagnosis was delayed and the disease was allowed to progress. One case showed increasing proteinuria with disease progression [5].
This case also shared clinical manifestations with LCPT, for example, crystal deposition within the proximal tubular epithelium and the presence of Fanconi syndrome [4]. In addition, the intraluminal cast formation composed of crystals had features reminiscent of the crystalline variant of myeloma cast nephropathy [11]. However, cases with both pathologic features are extremely unusual. Most cases of LCPT with crystals show very sparse intraluminal crystal formation because these crystal-forming light chains, unlike free light chains from most patients with light-chain cast nephropathy, have a low affinity for the Tamm–Horsfall glycoprotein [12]. The possibility exists that massive crystals can injure and rupture the tubular membranes and extravagate to the interstitium and intraluminal space [6].
In summary, we have presented a patient with multiple myeloma, Fanconi syndrome, and renal insufficiency. The cause was demonstrated to be extensive crystalline inclusions within proximal tubular epithelial cells and intraluminal cast formation in some tubules, as expected. However, our case was remarkable because despite the extensive crystalline inclusions in podocytes as well as glomerular mesangial and endothelial cells, clinical manifestations suggesting significant glomerular injury were absent. To achieve a better understanding of the discrepancy between these histologic findings and the typical clinical manifestations in crystalline nephropathy associated with multiple myeloma, further investigation is required.
References
2. Stokes M.B., Aronoff B., Siegel D., D'Agati V.D.. Dysproteinemia-related nephropathy associated with crystal-storing histiocytosis. Kidney Int 70:2006;597–602.


3. Heher E.C., Rennke H.G., Laubach J.P., Richardson P.G.. Kidney disease and multiple myeloma. Clin J Am Soc Nephrol 8:2013;2007–2017.



4. Sethi S., Fervenza F.C., Rajkumar S.V.. Spectrum of manifestations of monoclonal gammopathy-associated renal lesions. Curr Opin Nephrol Hypertens 25:2016;127–137.


5. Nasr S.H., Preddie D.C., Markowitz G.S., Appel G.B., D'Agati V.D.. Multiple myeloma, nephrotic syndrome and crystalloid inclusions in podocytes. Kidney Int 69:2006;616–620.


6. Gupta V., El Ters M., Kashani K., Leung N., Nasr S.H.. Crystalglobulin-induced nephropathy. J Am Soc Nephrol 26:2015;525–529.

7. Wang Y.D., Dong Z.Y., Zhang X.G., Zhang W., Yin Z., Qiu Q., Chen X.M.. Renal light chain deposition associated with the formation of intracellular crystalline inclusion bodies in podocytes: a rare case report. Intern Med 55:2016;369–373.


8. Jeon Y.L., Lee W.I., Choi Y., Kang S.Y., Kim M.H., Lim S.J., Lee S.H.. Crystalloid podocytopathy with focal segmental glomerulosclerosis in PCM: a case report. Diagn Pathol 10:2015;213



9. Matsuyama N., Joh K., Yamaguchi Y., Aizawa S., Kanai T., Kitajima T., Sakai O.. Crystalline inclusions in the glomerular podocytes in a patient with benign monoclonal gammopathy and focal segmental glomerulosclerosis. Am J Kidney Dis 23:1994;859–865.


10. Akilesh S., Alem A., Nicosia R.F.. Combined crystalline podocytopathy and tubulopathy associated with multiple myeloma. Hum Pathol 45:2014;875–879.


Figure 1
Mild wrinkling of capillary tufts and intraluminal material in tubules. Glomeruli show mild wrinkling of capillary tufts, yet otherwise unremarkable. Tubules focally display variable thickening of the basement membranes and an intraluminal pale-staining material (arrow) (light microscope, periodic acid-Schiff stain; original magnification 200×).
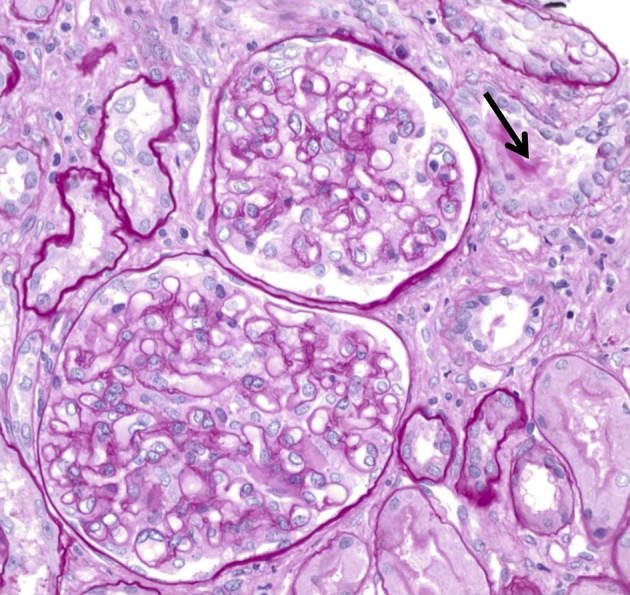
Figure 2
Crystalline inclusions in podocytes. Many oval or angulated crystalline inclusions are found in podocytes (electron microscope, original magnification 2,000×).
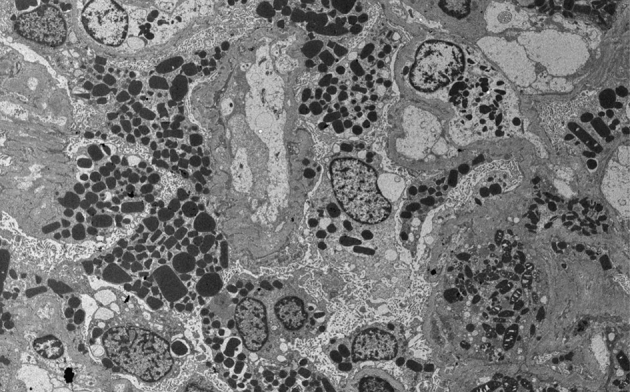
Figure 3
Intracellular crystalline inclusions in tubular epithelial cells. Tubular epithelial cells contain intracellular crystalline inclusions, which are also found in the luminal space (arrow) (electron microscope, original magnification 5,000×).

Figure 4
Intraluminal casts and crystalline inclusions in tubules. Some tubules contain intraluminal casts (arrow) composed of degenerated crystalline material. Crystalline inclusions similar to those found in cytoplasm are focally present along the periphery of the cast (big arrow) (electron microscope, original magnification 5,000×).

- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















