Introduction
Glomerular abnormalities in immunofluorescence and electron microscopic analysis have been noted in more than 50% of patients with cirrhosis while performing an autopsy and renal biopsy [1]. Although cirrhosis has been associated with a variety of glomerular lesions, mesangial proliferation with immunoglobulin A (IgA) deposition is frequently reported [1]. This cirrhosis-related IgA nephropathy (IgAN) is the most common form of secondary IgAN [1], [2].
Cirrhosis-related IgAN is usually clinically silent, and laboratory abnormalities have been reported in only 10ŌĆō20% of patients with cirrhosis [1]. Microscopic hematuria may occur, but gross hematuria rarely occurs [1], [3], [4]. Clinically evident renal failure is also uncommon [1], [5]. In particular, acute renal failure (ARF) is clearly exceptional in patients with IgAN secondary to hepatitis B virus (HBV)-associated cirrhosis, although ARF is a common occurrence in patients with cirrhosis [6], [7]. In contrast to idiopathic IgAN, in which ARF can occur during episodes of gross hematuria, cirrhosis-related IgAN rarely showed ARF associated with gross hematuria [1]. To our knowledge, acute tubular necrosis (ATN) associated with gross hematuria in HBV-associated cirrhosis has not been reported. We report, herein, an unusual case of ATN associated with gross hematuria in a patient with IgAN secondary to HBV-associated cirrhosis.
Case report
A 59-year-old woman was admitted to the hospital with the passage of dark urine, which had developed 7 days ago. She had no evidence of current or recent upper respiratory tract infection. She had been previously diagnosed with nonalcoholic HBV-related cirrhosis and chronic renal impairment. There was no familial history of liver or renal disease.
On admission, her body temperature was 36.5┬Ā┬░C and blood pressure was 117/65┬ĀmmHg. Her physical examination revealed periorbital swellings, ascites, pretibial pitting edema, and a maculopapular rash on both lower extremities. Laboratory investigations yielded the following measurements: hemoglobin, 10.9┬Āg/dL; white blood cells, 5,420/mm3; platelets, 71,000/mm3; prothrombin time, 16.2┬Āseconds; blood urea nitrogen, 24.8┬Āmg/dL; creatinine, 1.9┬Āmg/dL; sodium, 130┬ĀmEq/L; potassium, 3.8┬ĀmEq/L; chloride, 97┬ĀmEq/L; total bilirubin, 0.7┬Āmg/dL; alkaline phosphatase, 111┬ĀIU/L; ╬│-glutamyltransferase, 111┬ĀIU/L; aspartate aminotransferase, 37┬ĀIU/L; alanine aminotransferase, 9┬ĀIU/L; total protein, 6.1┬Āg/dL; albumin, 2.4┬Āg/dL; and globulin, 3.7┬Āg/dL. Urinalysis revealed blood 3+, protein 3+, red blood cell (RBC) casts, and dysmorphic RBC. A 24-hour urinary protein excretion was 2,640┬Āmg/day. Results of immunological studies revealed the following measurements: IgG, 1,828┬Āmg/dL; IgA, 655.9┬Āmg/dL; IgM, 204.9┬Āmg/dL; complement 3, 63.04 mg/dL; complement 4, 18.73┬Āmg/dL; and hemolytic complement 50, 11.8┬ĀU/mL. Antistreptolysin antibodies, cryoglobulins, and antineutrophil cytoplasmic antibody were negative. Antinuclear antibody was positive, but anti-dsDNA antibody was negative. Hepatic serologic testing was positive for hepatitis B surface antigen, hepatitis B envelope antigen, and hepatitis B envelope antibody. The HBV DNA titer was 4.19├Ś105┬Ācopies/mL. An abdominal ultrasound showed normal-sized kidneys, a coarse liver parenchyma with an irregular surface, moderate ascites, and splenomegaly. The ChildŌĆōPugh score was 8. The serum ascites albumin gradient was 1.26. A daily treatment with furosemide (40┬Āmg) and spironolactone (100┬Āmg) was initiated after a large-volume paracentesis was performed. On the 8th day of hospitalization, an upper gastrointestinal endoscopy was performed due to an anemia aggravation, which showed esophageal varices without any evidence of active bleeding. In order to prevent bleeding, a nonselective ╬▓-adrenergic blocker treatment was initiated, following an endoscopic variceal ligation. In addition, lamivudine treatment was started with an initial dose of 100┬Āmg once daily, which was continued for 8 months thereafter.
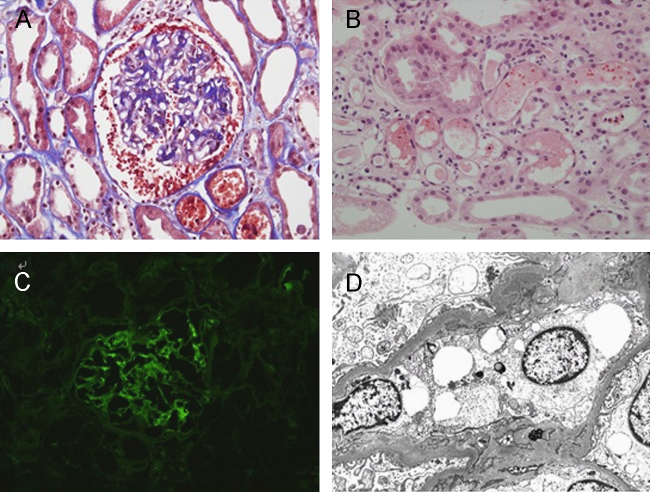
A percutaneous kidney biopsy was performed on day 13 because of the remarkable reduction in renal function and persistent gross hematuria. The serum creatinine level on day 13 was 4.4┬Āmg/dL. A light microscopic analysis revealed an increase in the mesangial matrix and proliferation of mesangial cells (Fig. 1A). There were no sclerotic glomeruli. RBC casts were seen in the renal tubules. Some renal tubules showed typical signs of tubular necrosis, such as tubular epithelial cell atrophy, loss of brush border, and regeneration of the epithelial cells (Fig. 1B). Immunofluorescence studies demonstrated diffuse mesangial deposits of IgA, IgM, and complement 3 (Fig. 1C). Electron microscopic examination revealed a few electron-dense deposits in the mesangial matrix, paramesangial deposits, and mildly effaced foot processes (Fig. 1D).
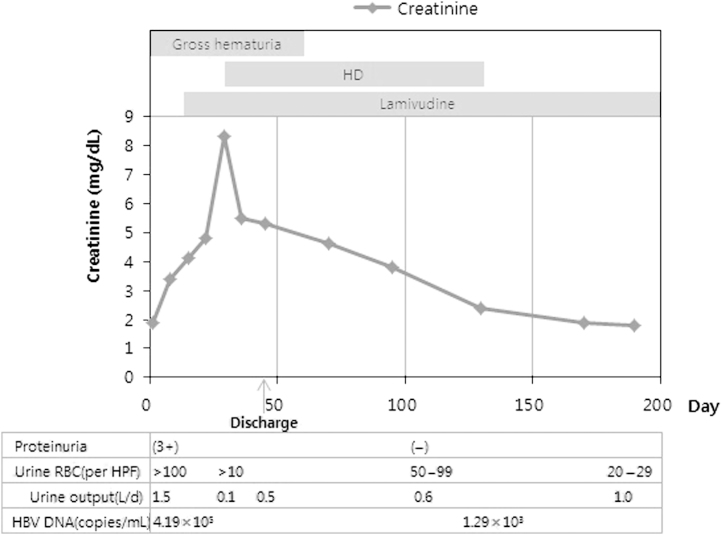
Hemodialysis was started on day 30 because the renal function continued to deteriorate, and the patient developed uremic symptoms. On day 30, the serum creatinine level was 8.5┬Āmg/dL and gross hematuria was noted. The patient was discharged on week 7. Regular hemodialysis was continued for approximately 3 months. Thereafter, hemodialysis was performed two times per week for 2 months, and then stopped. The serum creatinine level was 2.4┬Āmg/dL when hemodialysis treatment was stopped. Thereafter, renal function had completely returned to the baseline levels. Gross hematuria and proteinuria had disappeared by weeks 8 and 12 of onset, respectively, but microscopic hematuria continued to persist. After 8 months of treatment with lamivudine, the HBV DNA titer had decreased to 1.29├Ś103┬Ācopies/mL (Fig. 2).
Discussion
Mesangial IgA deposition associated with chronic liver disease is the most common form of secondary IgAN [1], [2]. In an autopsy series, predominant mesangial IgA deposits were found in 36% of the 75 patients with cirrhosis [8]. Although various causes of liver disease such as hepatitis B and C virus can induce IgAN, it occurs most commonly with alcoholic cirrhosis [1], [9]. However, pathogenesis of IgAN in advanced liver disease remains poorly understood. Impaired removal of IgA-containing complexes by the Kupffer cells in the liver is thought to predispose to IgA deposition in the kidney in patients with cirrhosis [10].
Unlike idiopathic IgAN, IgAN secondary to HBV-associated cirrhosis has usually no clinical signs of glomerular disease [1], [2], [9]. Microscopic hematuria and subnephrotic-range proteinuria may occur, but gross hematuria and overt nephrotic syndrome rarely occurs in spite of the frequency of glomerular IgA deposits in advanced liver disease [1], [2], [5], [9]. In addition, clinically evident renal failure is uncommon, although ARF is a common complication associated with cirrhosis [5], [6], [7]. ARF can occur during episodes of gross hematuria in patients with idiopathic IgAN [11]. ARF associated with gross hematuria is very uncommon in cirrhosis-related IgAN [1], [6]. To our knowledge, our case is the first report of ARF associated with gross hematuria in IgAN secondary to HBV-related cirrhosis.
In our case, ARF was preceded by gross hematuria with signs of glomerular bleeding such as RBC casts and a dysmorphic appearance of some red cells. A renal biopsy revealed typical evidence of ATN, such as distension of many renal tubules by intratubular red cells and tubular cell injury. These findings are similar to those described in an earlier study wherein idiopathic IgAN with ARF following bouts of gross hematuria has been reported [1]. Although the association of ATN with gross hematuria in patients with IgAN is not clearly understood, ATN may be induced by the iron released from lysed red cells in the tubules, possibly acting via the local generation of toxic oxygen-free radicals [11], [12].
Dialysis may be temporarily required in idiopathic IgAN patients with ARF and gross hematuria [11]. However, the serum creatinine concentration typically returns to baseline levels within several weeks/months, although there may be an incomplete recovery of renal function [11], [12]. Significant risk factors for the lack of complete recovery were duration of gross hematuria longer than 10 days, age greater than 50 years, decreased estimated glomerular filtration rate at baseline, and more severe tubular necrosis on renal biopsy [13]. In addition, very recently, Moreno et al. reported that older patients seemed to have longer duration of gross hematuria and recovery period [13]. In our case, despite the risk factors for the lack of complete recovery of renal function, the serum creatinine concentration returned to baseline levels within 5 months.
It has been recently reported that there was a complete remission of nephrotic syndrome by lamivudine therapy in HBV-associated membranoproliferative glomerulonephritis and membranous nephropathy in patients with cirrhosis [14], [15]. These previous reports suggested the possible role of lamivudine in the treatment of HBV-associated glomerulopathy. Although the possibility of spontaneous remission cannot be totally excluded, the resolution of proteinuria in parallel with rapid reduction in viremia level was most likely caused by lamivudine therapy.
In conclusion, our case is the first report of ARF associated with gross hematuria in a patient with IgAN secondary to HBV-related cirrhosis. Despite the risk factors for the lack of complete recovery of renal function, the patient's renal function and nephritic syndrome were completely recovered after the treatment with lamivudine. However, further studies are needed to investigate the beneficial effect of lamivudine therapy in IgAN secondary to HBV-related cirrhosis.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















