| Kidney Res Clin Pract > Volume 31(1); 2012 > Article |
|
Abstract
Background
Hemodialysis (HD) patients with functional iron deficiency often develop resistance to recombinant human erythropoietin (rhEPO). Recent studies have shown that intravenous ascorbic acid (IVAA) administration could override rhEPO resistance in HD patients. This study was undertaken to test the effects of IVAA in HD patients with normoferritinemic functional iron deficiency accompanied by EPO-hyporesponsive anemia.
Methods
Fifty-eight HD patients with normoferritinemic anemia (between 100 and 500┬Ā╬╝g/L) were included and divided into the control (N=25) and IVAA (N=33) groups. IVAA patients received 500┬Āmg of IVAA with each dialysis session for 3 months and an additional 4-month follow-up after the end of the therapy.
Results
Twenty patients had a response to IVAA with a significant increase in hemoglobin level (Hgb>1.0┬Āg/dL) and reduction of weekly rhEPO dosage compared with the control group after 3 months of treatment (P<0.05). Compared with non-responders, transferrin saturation (TSAT) was significantly decreased in the responders group (26┬▒11 vs. 35┬▒14%, P<0.05) on baseline data. There was a significant increase in serum iron and TSAT (baseline vs. 3 months, serum iron 57┬▒22 vs. 108┬▒22┬Ā╬╝g/dL, TSAT 26┬▒11 vs. 52┬▒7%, P<0.05) and a decrease in serum ferritin (377┬▒146 vs. 233┬▒145┬Āng/mL, P<0.05) in the responders group (N=20), but no significant changes in the control and non-responders groups (N=13) at 3-month treatment.
Conclusion
IVAA can be a potent and effective adjuvant therapy for HD patients with rhEPO-resistant normoferritinemic anemia. In addition, IVAA can reduce the dosage of rhEPO for anemia correction.
Keywords
Anemia, Erythropoietin, Hemodialysis, Vitamin CAnemia is one of the most important complications in chronic kidney disease (CKD) [1]. The pathogenesis of anemia in chronic kidney disease is multifactorial. The most important factor is inadequate production of erythropoietin (EPO), which is accompanied by a decline in renal function. Recombinant human erythropoietin (rhEPO) has been mainly used in the treatment of CKD-associated anemia [2], [3]. Despite the treatments with rhEPO or other erythropoiesis-stimulating agents (ESA), other factors affect the CKD-associated anemia, such as shortened erythrocyte survival, blood loss, iron and other nutritional deficiencies, hemolysis, the presence of uremic inhibitors of erythropoiesis, and inadequate iron stores [4].
Many patients with CKD have borderline iron deficiency, which may be the cause of resistance to EPO therapy [5]. Some patients with excessive iron stores may also exhibit CKD-associated anemia and are considered to have a functional iron deficiency state. Functional iron deficiency implies the state in which insufficient iron is released from the bone marrow. Functional iron deficiency cause insufficient erythropoiesis [6]. Failure of bone marrow erythropoiesis results in failure of iron utilization, characterized by high serum ferritin concentration, and low transferrin saturation (TSAT).
The potential role of adjuvant therapies in enhancing the efficacy of rhEPO in patients receiving maintenance dialysis has commanded attention in recent years [7], [8], [9]. Adjuvant therapies may help to reduce rhEPO requirements or allow dialysis patients to achieve increased hemoglobin (Hgb) concentrations, and derive more cost-effectiveness and greater clinical benefits from rhEPO treatment [8], [9], [10]. Currently, many studies imply that intravenous ascorbic acid (IVAA) treatment in hemodialysis (HD) patients improved Hgb and iron availability in EPO-hyporesponsive HD patients with iron overload. Vitamin C acts as an antioxidant, helps release iron from ferritin and mobilizes iron from the reticuloendothelial system to transferring [11]. Such effects of vitamin C help mobilize iron from tissue storage and increase iron utilization in erythropoiesis [12].
The purpose of the current study was to compare the efficacy of IVAA on improving anemia in EPO-hyporesponsive HD patients with normoferritinemia.
Participants were recruited from the dialysis center of Chosun University Hospital. The inclusion criteria were as follows: age older than 19 years, HD durationŌēź6 months, duration of rhEPO Ōēź6 months, and 6 consecutive months of stable ferritin, hematocrit, and rhEPO dose levels. All HD patients were selected based on the inclusion criteria: Hgb<11┬Āg/dL, hematocrit less than 33%, and serum ferritin 100ŌĆō500┬Ā╬╝g/L.
The exclusion criteria were as follows: previously diagnosed nonrenal cause of anemia other than iron deficiency; evidence of active or occult bleeding; blood transfusion during examination; history of malignancy, end-stage liver disease, or chronic hypoxia; and recent infection requiring antibiotics during examinations.
The prescription of HD was with polysulfone membranes and an average blood flow rate of 200ŌĆō300┬ĀmL/min. Kt/V was kept at 1.64┬▒0.18 during each treatment period. Patients received standard care and IVAA with each HD session. Five hundred mg of ascorbic acid mixed in 50┬ĀmL of 5% dextrose was infused intravenously for 30┬Āmin when each session was ended. rhEPO (Recormon, Seoul, Korea) was administered intravenously three times per week, and rhEPO dose was determined every month throughout the study according to the Kidney Disease Outcomes Quality Initiative guidelines. Maintenance iron replacement of 100┬Āmg as ferric oral iron (Feroba, Seoul, Korea) was administrated twice daily to all patients.
Informed consent was obtained from each patient before sample collection. Each patient's blood was collected before dialysis. Blood samples for measurement of Hgb, hematocrit, iron metabolism indices (serum iron, total iron binding capacity [TIBC], TSAT ratio (calculated by iron/TIBC├Ś100), serum ferritin), calcium, phosphorus, intact parathyroid hormone, and spKt/V were obtained at baseline, every month during the intervention period, and at 4 months after IVAA intervention. The C-reactive protein (CRP), aluminum, vitamin B12, folic acid, and vitamin C were measured at baseline, at 7 months. Responder of IVAA treatment was defined as an increase in Hgb of 1.0┬Āg/dL at 3 months.
Statistical analysis was performed using SPSS version 12.0 statistical package (SPSS Inc., Chicago, IL, USA). The values were expressed as mean┬▒standard deviation. An independent t test was used to compare the results between groups at baseline. Analysis of variance and multiple comparison test or paired t test was used for within-group comparison for pretreatment and posttreatment. The criterion for statistical significance was P<0.05.
A total of 58 patients were divided into a control group (25 patients) and IVAA group (33 patients). Both groups were similar in clinical data, sex, age, duration of HD, causes of end-stage renal disease, dry weight, ultrafiltration amount, and rhEPO dose at baseline (Table 1).
TSAT was significantly lower in the IVAA responders group than in the control and IVAA non-responders group (P<0.05). Baseline CRP levels tended to be lower in the IVAA responders group than the control and non-responders groups, but there was no statistical difference. Hgb, hematocrit, serum iron, TIBC, ferritin level, and the other factors showed no difference among the three groups (Table 2).
Hgb was significantly increased at 3 months and 7 months in the IVAA responders group compared with the control and IVAA non-responders groups (P<0.05, Fig. 1A). In the IVAA responders group, Hgb was increased from 9.3┬▒1.4┬Āg/dL at baseline to 11.2┬▒1.7┬Āg/dL at 3 months and maintained at 10.3┬▒1.5┬Āg/dL at 7 months. However, the control group (9.4┬▒1.9┬Āg/dL at baseline vs. 9.6┬▒0.9┬Āg/dL at 3 months vs. 9.7┬▒0.9┬Āg/dL at 7 months) and the IVAA non-responders group (9.8┬▒0.4┬Āg/dL vs. 9.8┬▒0.5┬Āg/dL vs. 9.8┬▒0.4┬Āg/dL) showed no improvement in Hgb levels.
Weekly requirement of rhEPO was significantly reduced at 3 months (89┬▒16┬ĀIU/kg/week) and 7 months (88┬▒19┬ĀIU/kg/week) compared with baseline values (143┬▒36┬ĀU/kg/week) in the IVAA responders group (P<0.05, Fig. 1B). In the IVAA non-responders group, weekly requirement of rhEPO was slightly reduced at 3 months and 7 months, but there was no significant difference. In the control group, the weekly requirement of rhEPO remained stable for 3 months of treatment and 4 months afterward (Fig. 1B).
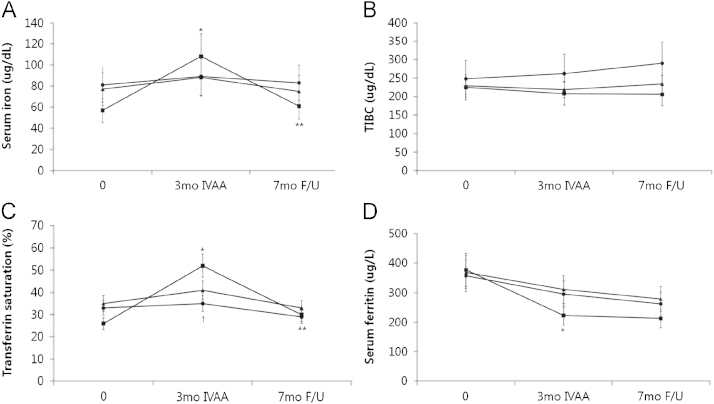
In the IVAA responder group, serum iron and TSAT were increased at 3 months and decreased at 7 months (57┬▒22┬Ā╬╝g/dL and 26┬▒11% at baseline, 108┬▒22┬Ā╬╝g/dL and 52┬▒7% at 3 months, and 61┬▒24┬Ā╬╝g/dL and 30┬▒13% at 7 months). Serum ferritin level was significantly decreased at 3 months (377┬▒146┬Āng/mL at baseline vs. 223┬▒145┬Āng/mL at 3 months, P<0.05, Fig. 2).
The serum iron, TSAT, TIBC, and serum ferritin of the control group and IVAA non-responders group had no significant changes during these periods.
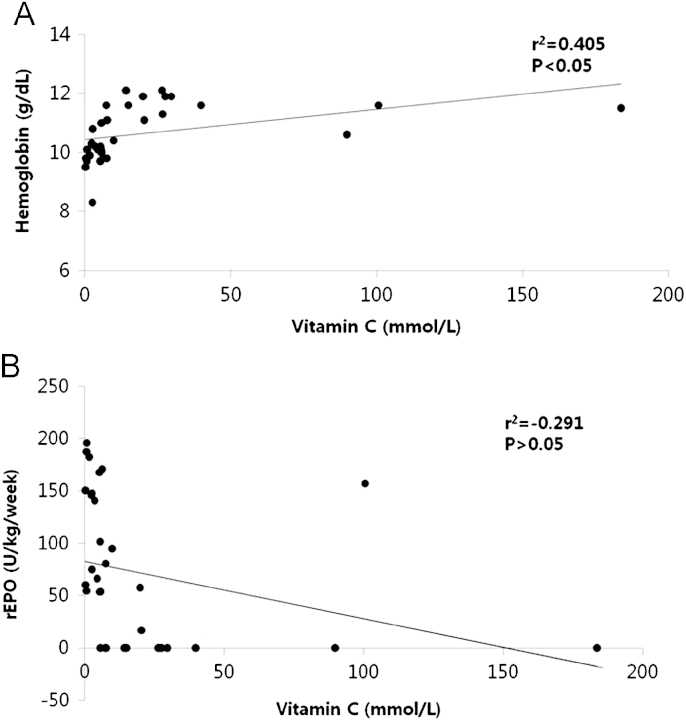
The relationship between the level of serum vitamin C and Hgb significantly showed a positive correlation (r=0.405, P<0.05). Serum vitamin C values and rhEPO dosage showed a negative correlation but no significant difference (P>0.05, Fig. 3).
In this prospective, randomized, double-blind study, we show that IVAA can be used as a potential adjuvant therapy for HD patients with EPO-resistant normoferritinemic anemia. Hgb was increased after IVAA administration, and weekly requirement of rhEPO declined simultaneously at 3 months. We also found that the serum iron and TSAT levels (57┬Ā╬╝g/dL and 26%, respectively, in this study) were therapeutic markers as vitamin C administration in HD patients with normoferritinemic anemia.
Anemia is a common complication that has remained one of the most significant and characteristic manifestations of CKD for more than 150 years [5], [13]. The use of bone marrow-stored iron has a critical role in CKD-associated anemia in patients on dialysis [14]. Inadequate iron storage and/or availability are the most common causes of suboptimal response to rhEPO treatment in HD patients [7], [15]. Functional iron deficiency occurs when the demand for iron exceeds the amount of tissue iron and erythropoiesis accelerates after rhEPO treatment. Functional iron deficiency is considered if TSAT and serum iron levels are reduced, and serum ferritin levels were more than 100┬Ā╬╝g/L. The patients were unable to utilize high levels of iron storage to improve their anemia [6], [14].
Vitamin C represents one of the most prominent antioxidants, exerting beneficial effects by inhibition of lipid peroxidation and by reducing endothelial dysfunction [16]. Another possible mechanism for a better response to EPO may be increased erythrocyte lifespan via both the prevention of Hgb denaturation and erythrocyte membrane lipid peroxidation [17]. It increases intestinal iron absorption and induces iron mobilization from inert tissue stores, including the reticuloendothelial system, and may improve iron availability [18]. It also has a role Hgb enzymatic incorporation of iron into protophorphyrin for heme synthesis [19]. In addition, most intervention studies found an increase of TSAT and/or a decrease in ferritin level in response to vitamin C [1], [3], [4]. Vitamin C deficiency can interfere with iron absorption and utilization [5], [12]. The effect of low vitamin C levels leads to a large amount of iron to be stored, with relatively insufficient utilization for erythropoiesis. Improved vitamin C status may lead to improvement of anemia in these patients [16]. Vitamin C supplementation leads to a significant increase in serum iron, indicating that it aids mobilization of stored iron in these patients. In this study, we found that the relationship between the level of serum vitamin C and Hgb significantly showed a positive correlation.
Chronic HD patients generally have lower plasma vitamin C levels. Vitamin C deficiency has complicated the management of dialysis patients since the initiation of renal replacement therapy, possibly caused by the loss of vitamin C during dialysis, increased consumption, or inadequate dietary intake [7], [12]. Normal plasma vitamin C levels in the nondialysis population are approximately 30ŌĆō60┬Āmmol/L. By contrast, plasma vitamin C in dialysis patients is frequently <10┬Āmmol/L, making the occurrence of scurvy a possible outcome. Vitamin C intake for dialysis patients is often restricted because of the avoidance of vitamin C-rich foods, and because of concerns about oxalosis [12], [16]. The vitamin C level of this study group was 9.7┬▒8.7┬Āmmol/L.
Tarng et al. [20] found IVAA administration to be effective in these cases. They studied HD patients with serum ferritin more than 500┬Ā╬╝g/L. All patients received 300┬Āmg of vitamin C, three times a week intravenously for 8 weeks. Afterward, Hgb increased from 8.8 to 10.7┬Āg/dL and rhEPO dosage decreased to 2/3. Keven et al. [21] studied HD patients without considering resistance to rhEPO and iron level. The patients received 500┬Āmg of vitamin C intravenously (three times per week). At the end, Hgb increased at least 1┬Āg/dL. The studies indicate a mechanism for the ability of high doses of vitamin C to mobilize iron for erythropoiesis, as has been suggested by several studies in rhEPO-resistant HD patients. Our work in normoferritinemic anemia HD patients was conducted after 3 months of ascorbic acid treatment. Patients with TSAT less than 25% had significantly increased levels of Hgb, hematocrit, TSAT, and significantly decreased rhEPO dose. In addition, Hgb and rhEPO dose did not change end of study follow-up evaluation was conducted 4 months after vitamin C treatment. However, serum ferritin levels were significantly decreased. These findings suggest that IVAA treatment facilitates iron release from storage sites and increases iron utilization in the erythropoiesis.
Our study has several limitations that should be considered. First, only a small number of patients were enrolled in the study. Further large, prospective, placebo-controlled studies are needed. Second, vitamin C treatment might be a risk for the development of oxalosis in HD patients. Although we did not evaluate oxalate levels, Tarng et al. [17] showed no significant increase in oxalate levels in IVAA treatment.
In conclusion, our study confirms that IVAA treatment can improve normoferritinemic anemia and low TSAT in HD patients. Further large-scale trials are needed.
References
1. Macdougall I.C.. Hyporesponsiveness to anemia therapyŌĆöwhat are we doing wrong? Perit Dial Int. 21:2001;S225ŌĆōS230.


2. Winearls C.G., Oliver D.O., Pippard M.J., Reid C., Downing M.R., Cotes P.M.. Effect of human erythropoietin derived from recombinant DNA on the anemia of patients maintained by chronic hemodialysis. Lancet 2:1986;1175ŌĆō1178.

3. Eschbach J.W., Egrie J.C., Downing M.R., Browne J.K., Adamson J.W.. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 316:1987;73ŌĆō78.


4. Attallah N., Osman-Malik Y., Frinak S., Besarab A.. Effect of intravenous ascorbic acid in hemodialysis patients with EPO-hyporesponsive anemia and hyperferritinemia. Am J Kidney Dis. 47:2006;644ŌĆō654.


5. Chan D., Irish A., Dogra G.. Efficacy and safety of oral versus intravenous ascorbic acid for anemia in hemodialysis patients. Nephrology 10:2005;336ŌĆō340.


6. Sezer S., ├¢zdemir F.N., Yakupoglu U., Arat Z., Turan M., Haberal M.. Intravenous ascorbic acid administration for erythropoietin-hyporesponsive anemia in iron loaded hemodialysis patients. Artif Organs 26:2002;366ŌĆō370.


7. Tarng D.C., Huang T.P., Wei Y.H.. Erythropoietin and iron: the role of ascorbic acid. Nephrol Dial Transplant. 16:2001;S35ŌĆōS39.

8. Tarng D.C.. Novel aspects of vitamin C in epoetin responses. J Chin Med Assoc. 70:2007;357ŌĆō360.

9. H├Črl W.H.. Is there a role for adjuvant therapy in patients being treated with epoetin? Nephrol Dial Transplant. 14:1999;S50ŌĆōS60.
10. Sharhrbanoo K., Taziki O.. Effect of intravenous ascorbic acid in hemodialysis patients with anemia and hyperferritinemia. Saudi J Kidney Dis Transplant. 19:2008;933ŌĆō936.
11. Tarng D.C., Huang T.P.. A parallel, comparative study of intravenous iron versus intravenous ascorbic acid for erythropoietin-hyporesponsive anaemia in haemodialysis patients with iron overload. Nephrol Dial Transplant. 13:1998;2867ŌĆō2872.


12. Handelman G.J.. Vitamin C neglect in hemodialysis: sailing between Scylla and Charybdis. Blood Purif. 25:2007;58ŌĆō61.


13. Stoian I., Manolescu B., Atanasiu V., Lupescu O.. New alternative for erythropoietin therapy in chronic renal failure. CE J Med. 2:2007;361ŌĆō378.
14. Handelman G.J.. Newer strategies for anemia prevention in hemodialysis. Int J Artif Organs 30:2007;1014ŌĆō1019.


15. Luciak M.. Antioxidants in the treatment of patients with renal failure. Rocz Akad Med Bialymst. 49:2004;157ŌĆō161.

16. Handelman G.J.. Vitamin C deficiency in dialysis patientsŌĆöare we perceiving the tip of an iceberg? Nephrol Dial Transplant. 22:2007;238ŌĆō331.

17. Candan F., Gultekin F., Candan F.. Effect of vitamin C and zinc on osmotic fragility and lipid peroxidation in zinc-deficient haemodialysis patients. Cell Biochem Funct. 20:2002;95ŌĆō98.


18. Bridges K.R., Hoffman K.E.. The effects of ascorbic acid on the intracellular metabolism of iron and ferritin. J Biol Chem. 261:1986;14273ŌĆō14277.


19. Goldberg A.. The enzymic formation of haem by the incorporation of iron into protoporphyrin: importance of ascorbic acid, ergothioneine and glutathione. Br J Haematol. 5:1959;150ŌĆō157.


Figure┬Ā1
Intravenous ascorbic acid (IVAA) treatment. Changes of hemoglobin concentration (a) and weekly recombinant erythropoietin (rhEPO) doses (b) after IVAA treatment during 3 months (mo) and posttreatment follow-up (F/U) at 4 months. Symbols: ŌŚÅŌ¢Ī, controls; Ō¢ĀŌ¢Ā, responders; Ō¢┤ non-responders. *P<0.05 vs. baselines; ŌĆĀP<0.05 vs. controls; #P<0.05 vs. responders.
non-responders. *P<0.05 vs. baselines; ŌĆĀP<0.05 vs. controls; #P<0.05 vs. responders.
 non-responders. *P<0.05 vs. baselines; ŌĆĀP<0.05 vs. controls; #P<0.05 vs. responders.
non-responders. *P<0.05 vs. baselines; ŌĆĀP<0.05 vs. controls; #P<0.05 vs. responders.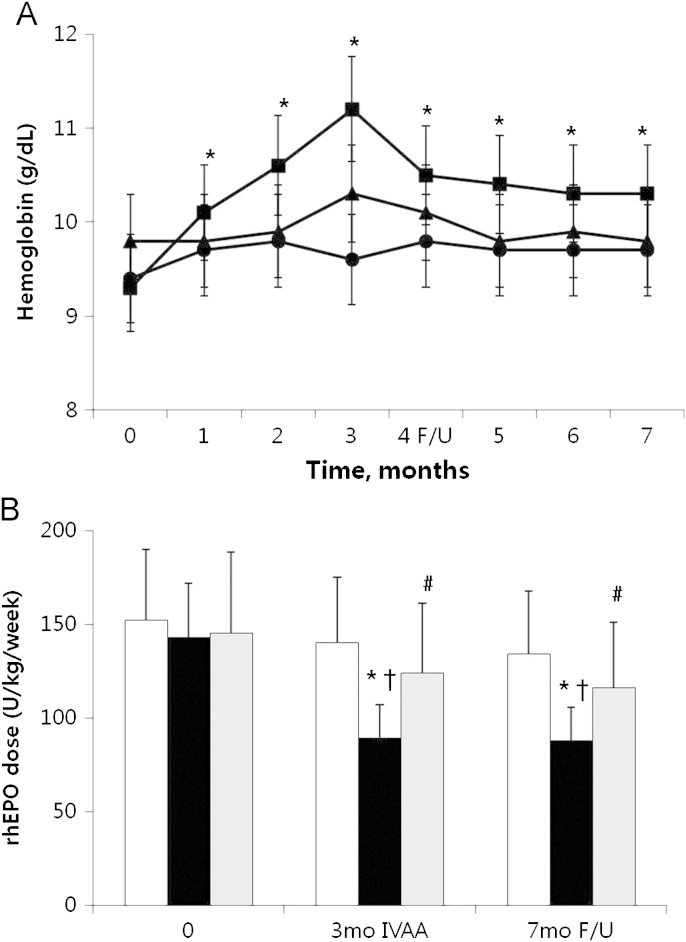
Figure┬Ā2
Effects of intravenous ascorbic acid and posttreatment follow-up. Serum iron (a), TIBC (b), transferrin saturation (c) and serum ferritin (d). Symbols: ŌŚÅ, controls; Ō¢Ā, responders; Ō¢┤ non-responders. *P<0.01 vs. baselines; ŌĆĀP<0.01 vs. responders; **P<0.01 vs. at 3 months.
Figure┬Ā3
Correlation between serum vitamin C level and weekly recombinant erythropoietin (rhEPO) dosage, hemoglobin in patients undergoing intravenous ascorbic acid treatment.
Table┬Ā1.
Baseline Characteristics
Table┬Ā2.
Baseline Laboratory Data of Control and Intravenous Ascorbic Acid Treated Patients
| Control (N=25) | IVAA group (N=33) | IVAA responders (N=20) | IVAA non-responders (N=13) | |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 9.4┬▒0.9 | 9.5┬▒1.1 | 9.3┬▒1.4 | 9.8┬▒0.4 |
| Hematocrit (%) | 28.2┬▒2.8 | 27.5┬▒3.0 | 27.2┬▒3.7 | 28.2┬▒1.5 |
| WBC (103/╬╝L) | 5892┬▒1560 | 5942┬▒1951 | 6269┬▒2298 | 5440┬▒1389 |
| Platelet (103/╬╝L) | 204┬▒78 | 193┬▒54 | 200┬▒54 | 181┬▒57 |
| Serum iron (╬╝g/dL) | 81┬▒25 | 65┬▒25 | 57┬▒22 | 77┬▒22 |
| TIBC (╬╝g/dL) | 248┬▒66 | 227┬▒45 | 225┬▒52 | 229┬▒38 |
| Transferrin saturation (%) | 33┬▒10 | 30┬▒13 | 26┬▒11ŌüÄ | 35┬▒14 |
| Serum ferritin (ng/mL) | 357┬▒105 | 374┬▒129 | 377┬▒146 | 369┬▒103 |
| Transferrin (g/L) | 1.6┬▒0.3 | 1.7┬▒0.3 | 1.7┬▒0.3 | 1.7┬▒0.3 |
| Total calcium (mg/dL) | 8.5┬▒0.7 | 9.4┬▒1.0 | 9.2┬▒0.6 | 9.8┬▒1.3 |
| Phosphorus (mg/dL) | 4.2┬▒1.1 | 4.9┬▒1.3 | 5.2┬▒1.2 | 4.8┬▒1.4 |
| Intact PTH (pg/mL) | 261┬▒219 | 312┬▒443 | 349┬▒517 | 255┬▒350 |
| Cholesterol (mg/dL) | 149┬▒39 | 142┬▒33 | 134┬▒22 | 153┬▒46 |
| Serum albumin (g/dL) | 4.3┬▒0.3 | 4.1┬▒0.2 | 4.1┬▒0.2 | 4.0┬▒0.2 |
| Predialysis BUN (mg/dL) | 71┬▒14 | 76┬▒17 | 81┬▒17 | 69┬▒13 |
| Urea reduction rate (%) | 75.5┬▒5.9 | 74.9┬▒5.5 | 73.8┬▒5.4 | 76.7┬▒5.6 |
| spKt/V | 1.7┬▒0.3 | 1.7┬▒0.3 | 1.7┬▒0.3 | 1.7┬▒0.3 |
| nPCR (g/kg/day) | 1.3┬▒0.3 | 1.3┬▒0.3 | 1.3┬▒0.3 | 1.3┬▒0.3 |
| C-reactive protein (mg/dL) | 0.8┬▒1.1 | 0.5┬▒0.4 | 0.4┬▒0.2 | 0.6┬▒0.7 |
Values are expressed as mean┬▒standard deviation. BUN, blood urea nitrogen; IVAA, intravenous ascorbic acid; nPCR, normalized protein catabolic rate; TIBC, total iron binding capacity. spKt/V reflects the dialysis single-pool kinetics. K is the dialyzer ultrafiltration coefficient, t is the time, and V is the volume.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















