Very low protein diet plus ketoacid analogs of essential amino acids supplement to retard chronic kidney disease progression
Article information
Abstract
Background
A very low protein diet (VLPD) with ketoacid analogs of essential amino acids (KA/EAA) administration can remarkably influence protein synthesis and metabolic disturbances of patients with advanced chronic kidney disease (CKD), and may also slow the decline in renal function.
Methods
A retrospective cohort study was carried out to monitor renal progression and metabolic and nutritional status among 140 patients with CKD stage III or IV. One group (n = 70) was on a low protein diet (LPD) with 0.6 g of protein intake, and another group (n = 70) was on a VLPD with 0.3 g of protein and KA/EAA supplementation of 100 mg/kg/day for 12 months.
Results
At 12-month follow-up, estimated glomerular filtration rate (GFR) significantly decreased from 41.6 ± 10.2 to 36.4 ± 8.8 mL/min/1.73 m2 (P < 0.001) and urine protein increased from 0.6 ± 0.5 to 0.9 ± 1.1 g/day (P = 0.017) in the LPD group, but no significant changes in estimated GFR and urine protein were found in the VLPD plus KA/EAA group. A significant mean difference in rate of change in estimated GFR (−5.2 ± 3.6 mL/min/1.73 m2 per year; P < 0.001) was observed between the two groups. After Cox regression analysis, treatment with VLPD plus KA/EAA significantly protected against the incidence of declining GFR > 10% annually (adjusted hazard ratio, 0.42; 95% confidence interval, 0.23–0.79; P = 0.006) and significant correlations were found between using VLPD plus KA/EEA and increased GFR.
Conclusion
VLPD supplementation with KA/EAA is associated with delayed renal progression while preserving the nutritional status in the patients with CKD. Co-administration of VLPD and KA/EAA may prove an effective alternative to conservative management of CKD.
Introduction
Ketoacid analogs of essential amino acids (KA/EAA) are prescribed in predialysis patients with chronic kidney disease (CKD) to lower generation of toxic metabolic products and improve nutritional status [1,2]. Because KA/EAA lack the amino group bound to the alpha carbon of an amino acid, a KA/EAA diet could convert their respective amino acids without providing additional nitrogen. The beneficial effects of protein restriction supplemented with ketoacid on the progression of CKD are multifactorial and may include decreasing nitrogen waste, oxidative stress and inflammatory response including transforming growth factor-beta and protection against hemodynamic changes in glomerular hyperfiltration [3–5]. Among patients with CKD, ingestion of oral-specific renal formula may require an increase in dietary energy and fiber intake as well as decreased dietary protein intake [6].
Dietary protein plays an important role in the progression of CKD, and a low protein diet (LPD) is recommended to patients with advanced CKD to slow the decline in glomerular filtration rate (GFR) [7]. Additionally, several clinical trials and recent meta-analysis have documented that very low protein diets (VLPD) supplemented with KA/EAA preserve the rate of progression of advanced CKD [8–10] and delay the need for long-term dialysis treatment among patients with advanced CKD [11,12]. However, an MDRD study indicated that only a prescribed dietary protein intake of 0.6 g/kg/day, but not VLPD plus KA/EAA treatment, reduced the decline in GFR among patients with advanced CKD with GFR less than 25 mL/min/1.73 m2 [13]. Moreover, in long-term follow-up of the MDRD study, VLPD plus KA/EAA treatment did not delay progression to dialysis or transplantation [14]. Consequently, no definitive conclusions have been reached on the role of protein-restricted regimens plus ketoacid analogs in slowing the loss of GFR among patients with CKD stage III, particularly in the era of more vigorous blood pressure with renin angiotensin aldosterone system inhibitors and tight glycemic control [15]. This retrospective study was designed to test the hypothesis that administering VLPD plus KA/EAA would decrease renal progression among patients with CKD compared with treatment by LPD.
Methods
The patient’s data from August 2010 and January 2015 were collected retrospetively at the Phramongkutklao Hospital in Bangkok, Thailand. The study was approved by the Institutional Review Board (R089h/57) of Phramongkutklao Hospital.
Subjects
Inclusion criteria were age 18 years or older and nondialysis patients with CKD stage III or IV. Only patients with regular follow-up based on medical reports and prescriptions that had been recorded in our hospital electronic database for a period of 12 months before the study were recruited for the screening visit. Patients with active malignancy, severe heart, lung or liver disease, stroke, chronic infection, protein-energy wasting based on anthropometric data and laboratory data, especially serum albumin < 3.5 g/dL, pregnancy, any immunological or inflammatory disorders or known history of KA/EAA hypersensitivity were excluded from the study.
All patients with CKD received multidisciplinary care team treatment including renal dietitian consultations. According to the standard of care, patients with CKD stage III or IV were followed in nephrology clinics every three months. Nutritional compliance with dietary protein intake was measured using a diet record and analyzed using standard food composition tables. According to the medical history records, eligible patients were divided in two groups based on their regimen 12 months before the study as described below. The treatment group (n = 70) was prescribed a VLPD of 0.3 g of protein and KA/EAA 100 mg/kg/day and the control group (n = 70) was prescribed an LPD of 0.6 g of protein and no KA/EAA. The KA/EAA used in our study was Ketosteril (Fresenius Kabi, Bad Homburg vor der Höhe, Germany), 630 mg/tablet prescribed at 1 tablet/5 kg body weight daily. The prescriptions were made by the nephrologists monitoring the patients in the predialysis period to maintain the nutritional biochemical parameters.
The primary outcome was the differences in estimated GFR decline rates between the treatment and control groups. The secondary outcomes were the differences of the amount of proteinuria that was evaluated using urine protein-to-creatinine ratio and biochemical profiles between the two groups. Patients with significant CKD progression were defined as estimated GFR decline > 10% annually from baseline.
Clinical and laboratory monitoring
Medical history, physical examinations and biochemical tests were taken at the first visit before starting treatment. All routine laboratory tests, including assays for plasma levels of hemoglobin, albumin, potassium, creatinine, calcium, phosphate and estimated GFR using the 2009 CKD-EPI creatinine equation and staging according to The Kidney Disease: Improving Global Outcomes (KDIGO) 2012, were performed at baseline and at the end of the study. Adverse events that were or were not considered related to treatment were monitored from medical data sheets.
Statistical analysis
Measured values of the results were expressed in mean with standard deviation and percentage. The paired t test was used to compare the change of parameters within group at baseline and 12 months. Parameters were compared between groups at baseline and 12 months using the chi-square test, Fisher’s exact test and Student t test. ANCOVA analyses with baseline value of treatment period as covariate were used to assess for between-group mean differences at 12 months. We used Cox regression to analyze the association between declining GFR and VLPD with KA/EAA administration, unadjusted and then adjusted for age, diabetes, hypertensive nephropathy, body mass index (BMI), systolic blood pressure, baseline GFR, baseline proteinuria, angiotensin converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) and statin treatment. Additionally, we used multivariate regression to analyze the association between change of GFR and VLPD with KA/EAA administration after adjusting for other confounding factors. Statistical analyses were performed using the SPSS version 15 program for Windows (SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered statistically significant.
Results
A total of 282 patients with CKD stage III or IV were screened for possible study enrollment. One hundred forty patients were eligible according to the entry criteria and received either LPD or VLPD plus KA/EAA treatment. In the LPD and VLPD plus KA/EAA groups, average age was 68.4 ± 10.6 and 73.7 ± 9.3 years, respectively (P = 0.002), and male prevalence was 44.9% and 27.1%, respectively (P = 0.029). Type 2 diabetes prevalence differed in the two groups (P = 0.042), being 61.4% and 44.3% in the LPD and VLPD plus KA/EAA groups, respectively. Prescription medications including ACEI/ARB (61.4% vs. 41.4%, P = 0.018) and statins (84.3% vs. 60%, P = 0.001) were found to be higher in LPD group. The average dose of KA/EAA in VLPD treatment was 11.2 ± 1.3 tablets per day. Characteristics of the patients are shown in Table 1. No significant differences were found in body weight, systolic blood pressure, primary renal diseases, the amount of the proteinuria, hemoglobin, serum potassium, bicarbonate or calcium, but significantly increased BMI, diastolic blood pressure and serum phosphate were found in the LPD group.
Renal function and proteinuria after treatments
In the LPD and VLPD plus KA/EAA groups, average estimated GFR was 41.6 ± 10.2 and 39.1 ± 9.2 years, respectively (P = 0.131). The VLPD plus KA/EAA group exhibited no significant change in estimated GFR levels from 39.1 ± 9.2 mL/min/1.73 m2 at baseline to 38.9 ± 12.0 mL/min/1.73 m2 at 12 months (Table 2), but the LPD group with or without ACEI/ARB presented a significant change in estimated GFR levels from 41.6 ± 10.2 mL/min/1.73 m2 at baseline to 36.8 ± 8.8 mL/min/1.73 m2 at 12 months (P < 0.001) (Table 2). GFR levels during the 12-month follow-up declined on average by −5.2 ± 3.6 mL/min/1.73 m2 in the LPD group and by −0.3 ± 6.8 mL/min/1.73 m2 in the VLPD plus KA/EAA group (P < 0.001) (Table 3, Fig. 1). A significant mean difference in rate of change in estimated GFR (−5.2 ± 3.6 mL/min/1.73 m2 per year; P <0.001) was observed between the two groups. Similarly, these results were found in the change of blood urea nitrogen (22.8 ± 7.3 vs. 24.3 ± 7.1 mg/dL, P = 0.031) and serum creatinine (1.6 ± 0.4 vs. 1.8 ± 0.4, P < 0.001) in the LPD group after 12 months of treatment. Moreover, the LPD group with or without ACEI/ARB showed a significant increase in urine protein levels from 0.6 ± 0.5 g/gCr at baseline to 0.9 ± 1.1 g/gCr at 12 months (P = 0.017) (Table 2, Fig. 1), but no significant difference was observed in mean change in urine protein between the two groups (Table 3).
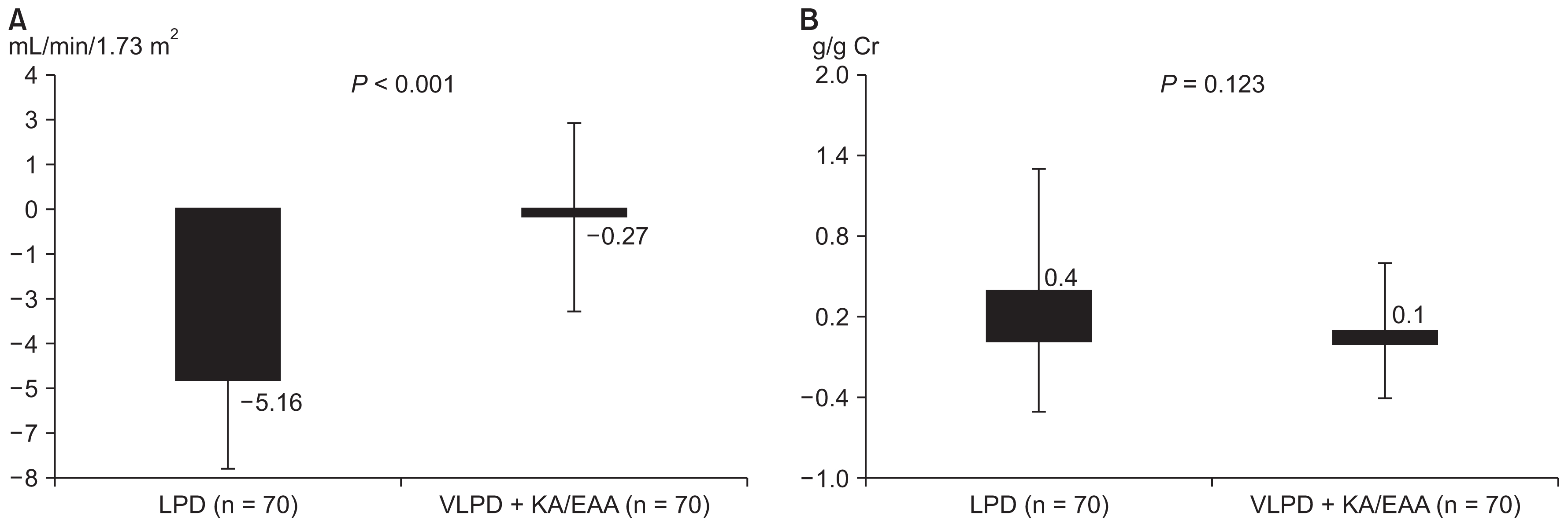
Renal function and proteinuria after 12 months of treatment
Mean changes of estimated glomerular filtration rate (A) and proteinuria (B) presented a significantly worsening progression in the low protein diet (LPD) group (P < 0.05) compared with the very LPD (VLPD) + ketoacid analogues of essential amino acids (KA/EAA) group.
Table 4 demonstrates the results of Cox regression analysis of significant GFR decline with some potential confounding parameters. The risk for GFR decline > 10% annually was lower with VLPD plus KA/EEA compared with LPD (hazard ratio [HR], 0.48; 95% confidence interval [CI] 0.28–0.82; P = 0.008). This protective effect was independent of age, diabetes, hypertensive nephropathy, ACEI/ARB, statins, BMI, systolic blood pressure, baseline GFR and proteinuria (adjusted HR, 0.42; 95% CI, 0.23–0.79; P = 0.006).
Univariate analysis was performed to assess the relationship between clinical parameters with change of GFR. VLPD plus KA/EEA compared with LPD was positively correlated with increased estimated GFR, whereas diabetes, baseline estimated GFR, and proteinuria were negatively correlated with increased estimated GFR. After multiple regression analyses, significant correlations were found among age, baseline estimated GFR, BMI, and use of VLPD plus KA/EEA with change in GFR (Table 5).
Changes in metabolic profiles and electrolytes after treatment
During the 12-month study, the LPD group exhibited significantly increased plasma potassium and decreased plasma phosphate (P < 0.001), but no significant difference was observed regarding mean changes in serum albumin, hemoglobin, potassium, bicarbonate, calcium and phosphate levels between the two groups (Table 5). No serious complications related to protein intake were observed and none of the patients received acute dialysis during the study.
Discussion
The present study constitutes a retrospective clinical trial of VLPD plus KA/EAA supplementation on renal function among patients with CKD stage III or IV. VLPD, when supplemented with KA/EAA, could decrease the rate of CKD progression among these patients when compared with LPD treatment through a reduced dietary protein intake and controlled metabolic waste products during CKD progression.
Decreased dietary protein intake was associated with a retardation of GFR loss and reduction in proteinuria among predialysis patients with CKD [16–18]. However, it exposed patients to the risk of protein malnutrition. In experimental CKD models, high protein intake induces renal proinflammatory cytokines, profibrotic growth factors, oxidative stress, glomerular hyperfiltration and glomerular hypertrophy, and results in glomerular sclerosis, mesangial matrix expansion and proteinuria [19]. Inversely, LPD supplemented with ketoacids was more effective than LPD alone in protecting kidney function from oxidative stress injury in remnant kidney tissue [20]. As a consequence, VLPD with KA/EAA has been supposed to protect against the progression of CKD and preserve residual renal function, slowing the GFR decline toward end-stage renal disease [21]. Strategies to avoid protein malnutrition include supplementing decreased protein intake with essential amino acids for CKD populations. Our results showed a better preservation of GFR over 12 months among patients that were prescribed VLPD supplemented with KA/EAA, but not among those that were prescribed LPD alone. Similar to related retrospective, prospective, or randomized studies, VLPD plus KA/EAA preserved GFR and delayed the need for long term dialysis treatment among patients with advanced CKD [8–12]. Recently, a well-defined, randomized, controlled trial demonstrated that vegetarian VLPD supplemented with ketoacid analogs delayed dialysis initiation among patients with advanced CKD with GFR less than 20 to 30 mL/min/1.73 m2 by ameliorating metabolic disturbances [22]. Finally, recent meta-analyses and real clinical practice in nephrology registry supported that VLPD or LPD, supplemented with ketoacid analogs, significantly prevented the deterioration of GFR [10,23,24]. Related clinical trials were investigated among patients with very late stages of CKD or CKD stage V, who wished to defer dialysis treatment. However, our study included CKD stage III with an average GFR of 40 mL/min/1.73 m2. Therefore, the positive role of VLPD plus KA/EAA treatment might become more apparent in CKD stage III without dialysis.
Our results did not demonstrate a significant change in proteinuria after 12 months of treatment. However, administration of VLPD plus KA/EAA tended to lower proteinuria levels, while LPD treatment significantly increased proteinuria levels. Related studies have indicated an association between a restricted protein diet with or without KA/EAA and reduced proteinuria levels among patients with CKD [25–27]. The anti-proteinuric response to restricted dietary protein intake was reported among patients with significant proteinuria levels > 1 g/day and high baseline serum phosphate [25–27]. The beneficial effects of KA/EAA treatment on proteinuria was limited in our study because of the differences in patient population, especially low baseline urine protein and normal serum phosphate levels among patients in our study.
VLPD supplemented with ketoacid analogs may serve a role in the conservative management and improved metabolic profiles among patients with CKD. Current evidence indicates that restricted dietary protein intake improves metabolic surrogates of CKD, including azotemia, bone and mineral disorder and acidosis [28]. Related studies have shown higher serum bicarbonate, higher serum calcium and lower phosphorus levels among those receiving VLPD plus KA/EAA compared with LPD [22,29]. However, we did not observe any significant differences in changes of bicarbonate, calcium and phosphate levels between VLPD and LPD groups, because of different population settings, especially in CKD stage III with normal metabolic disturbances at baseline.
This study had several limitations. First, the patient selection was not random and could have contained selection bias, including diet compliance, other nephroprotective therapies, sodium restriction and optimal glycemic control. However, factors affecting renal progression other than dietary protein intake were included and analyzed before and during the study and the confounding factors were considered in multiple regression analysis. Secondly, this study included a relatively small number of patients and short follow-up time. The short follow-up time may explain the nonsignificant differences in main renal outcomes, including renal replacement therapy. Thirdly, dietary protein intake was assessed using a three-day food diary, so we cannot exclude the possibility of some patients misjudging intake. As a retrospective study using medical electronic databases, we were unable to confirm whether patients actually took the dispensed medications. Finally, the generalizability of our data is limited to a CKD population with advanced age at 70 years. These results may not be applicable to all patients with CKD.
In conclusion, VLPD plus KA/EAA treatment was associated with even greater preservation of renal function in CKD stage III–IV. KA/EAA supplementation provides an additional therapeutic intervention to slow CKD progression. The effect of this treatment on long-term renal function among patients with CKD should be further assessed using larger patient populations and longer treatment periods.
Acknowledgments
The authors wish to acknowledge the contributions of the following individuals to this study: staff in the Division of Nephrology and Biomedical Clinical Research Center in Phramongkutklao Hospital.
Notes
Conflicts of Interest
This study was supported by the Department of Medicine, Phramongkutklao Hospital and College of Medicine. All authors have no conflicts of interest to declare.




