Predictive parameters of arteriovenous fistula maturation in patients with end-stage renal disease
Article information
Abstract
Background
The objevctive of the present study was to explore the potential influence of blood markers and patient factors such as risk factors, kidney function profile, coagulation profile, lipid profile, body mass index, blood pressure, and vein diameter on the maturation of arteriovenous fistula (AVF) in patients with end-stage renal disease.
Methods
Retrospective data from 300 patients who had undergone AVF creation at the Royal Infirmary of Edinburgh were examined. A predictive logistic regression model was developed using a backward stepwise procedure. Model performance, discrimination, and calibration were assessed using the receiver operating characteristic (ROC) curve and Hosmer–Lemeshow goodness-of-fit test. The final model was externally validated by 100 prospective patients who received a new fistula at the Royal Infirmary of Edinburgh.
Results
A total of 400 (300 retrospective and 100 prospective) patients were recruited for this study, with a mean age of 60.14 ± 15.9 years (development set) and 58 ± 15 years (validation set), respectively (P = 0.208). Study results showed that males were twice as likely to undergo fistula maturation as females, while patients with no evidence of peripheral vascular disease (PVD) were three times more likely to mature their fistula and a preoperative vein diameter > 2.5 mm resulted in a fivefold increase in fistula maturation as compared with a vein size of less than 2.5 mm. The model for fistula maturation had fair discrimination, as indicated by the area under the ROC curve (0.68), but good calibration as indicated by the Hosmer–Lemeshow test (P = 0.79). The area under the receiver operating curve for the validation model in the validation set was 0.59. Similarly, in the validation set, the Hosmer–Lemeshow statistic indicated an agreement between the observed and predicted probabilities of maturation (P > 0.05).
Conclusion
Gender, PVD, and vein size are independent predictors of AVF maturation. The clinical utility of these risk categories in the maturation of AVF requires further evaluation in longer follow-up.
Introduction
Chronic kidney disease (CKD) is an important condition with considerable public health implications that are further multiplied by rising rates of diabetes mellitus, obesity, and hypertension and the aging of the population [1]. The recorded prevalence of CKD for 2014 to 2015 in England was 4.1 per 100 people among those aged 18 years and older versus 3.16 per 100 people aged 18 years and older in Scotland [2]. A total of 58,968 adult patients were reported as receiving renal replacement therapy in the United Kingdom (UK) at the end of 2014 for a prevalence of 913 per million people in the UK [3].
End-stage renal disease (ESRD) is a serious condition affecting a large part of the population and, as such, represents a considerable financial burden for the health sector. After kidney transplantation, dialysis is the best active treatment option for people with ESRD [4]. To ensure that dialysis therapy can be efficiently performed, all patients need a fully developed fistula that is appropriate for the process of cannulation. In order to achieve this, several stages have to be covered, namely, pre-ESRD treatment by a kidney specialist and early referral to vascular surgeons for the construction, development, and cannulation of a fistula by a dialysis specialist. Vascular surgeons are required to construct mature arteriovenous fistulas (AVFs) so as to ensure their successful maturation and proper functioning to withstand dialysis. AVFs are cheaper; last longer; have lower infection, morbidity, and mortality rates; and reduce the chances of repeat intervention [5,6]. However, access problems represent the main determinant of morbidity among hemodialysis patients and put a considerable degree of financial pressure on the health care sector [3]. Asif et al [7] indicated that the percentage of AVFs that fail to develop sufficiently for dialysis is between 28% and 53%. Dialysis therapy is often postponed for up to half a year or more to allow for extra time for the fistula to develop; a fistula that fails to mature is a fistula that either has never matured so as to be useful, is difficult to cannulate, or that fails to generate the necessary blood flow (600 mL/min) for successful two-needle dialysis [8].
It may be possible to improve the end results of AVF by gaining a more comprehensive picture of the factors involved in the maturation of fistulas. In addition, such analysis can provide important information during the presurgical evaluation that surgeons can use to make their decisions. Independent predictive factors may be beneficial in anticipating the rate of fistula maturation without the use of invasive tests and could be cost-effective. The main purpose of the present study therefore was to explore the potential influence of blood markers and patient characteristics such as risk factors, kidney function profile, coagulation profile, lipid profile, body mass index (BMI), blood pressure (BP), and vein diameter on the maturation of AVF in patients with ESRD.
Methods
Research design
The study protocol was approved by the South East Scotland Research Ethics Committee 03, Edinburgh, UK (no. 09/S1103/29), and the favorable ethical opinion was obtained from the Queen Margaret University (Edinburgh, UK) ethics committee for the present study, which was performed in two stages. The development stage involved the collection of retrospective data and the identification of relevant independent predictors for a predictive model of fistula maturation. The second (validation) stage involved the validation of a development model on a prospective set of data from the Royal Infirmary of Edinburgh.
Development stage
Using the retrospective clinical database of patients with ESRD, researchers identified 398 patients who had undergone vascular access surgery for the first time for AVF creation between February 2007 and October 2010. Patients who underwent a repeat AVF creation (second and further fistula creations) were not included in the present study. Patients whose records contained incomplete information (n = 98) in relation to fistula outcomes were also excluded from the study. The data of the 300 patients pinpointed for inclusion were obtained from patient medical records and surgery records; specifically, information on patient age, gender, and risk factors; peripheral vascular disease (PVD); diabetes mellitus; hypertension; smoking (ever versus never); and dialysis (ever versus never) was sourced. Patients were defined as obese when their BMI was > 30 kg/m2, consistent with the World Health Organization classification [9]. Fistula characteristics were ascertained, included fistula type and location (i.e., right or left). Clinically important biomedical factors including estimated glomerular filtration rate (eGFR), creatinine, blood urea, serum potassium, sodium, calcium, bicarbonate, prothrombin time (PT), international normalized ratio (INR), high-density lipoprotein, triglycerides (TG), total cholesterol (TC), and vein diameter were also included in the analysis.
Validation stage
A total of 168 patients who had undergone surgery for AVF at the Royal Infirmary of Edinburgh between the years 2010 and 2012 were contacted for prospective clinical research. Out of the 168 patients contacted for this trial, 23 were unwilling to participate in this trial, 10 dropped out during follow-up, and 35 were excluded from the study because they did not meet the inclusion and exclusion criteria. The most often cited reason for a patient not being willing to participate was personal commitments. Moreover, excluded patients included non-native English speakers (who were unable to give informed consent) and those who had undergone a second attempt of AVF creation. Patients who had undergone a repeat AVF creation (second and further fistula creations) were not included in this study. Thus, a total of 100 patients were finally recruited into the prospective study. Written informed consent was obtained from the patients at the time of vascular imaging in the vascular lab at the Royal Infirmary of Edinburgh. Patients with current unstable angina or uncontrolled diabetes and a known bleeding disorder (i.e., bleeding diathesis) were excluded from the analysis.
Duplex investigation of the veins was performed to measure the diameter of arm veins according to a standard protocol by vascular scientists in the vascular clinic of the Royal Infirmary of Edinburgh. All procedures such as general physical examination consisted of inspection and palpation of the vessels of the upper arm and forearm and measurement of brachial artery BP. Subsequently, height and weight details were recorded in order to calculate the BMI values and blood samples were obtained by the regular NHS staff. PVD was identified through a physical examination and by comparing the BP in the arm and ankle. An ankle-brachial pressure index value ≤ 0.90 reliably elucidates 95% of symptomatic arteriogram-positive PVD individuals and almost 100% of healthy controls [10].
Outcomes definition
Failure-to-mature fistula is defined as a fistula that has never matured so as to be useful, is difficult to cannulate, or that fails to generate the necessary blood flow (600 mL/min) for a successful two-needle dialysis [8]. Maturation was defined as the ability of AVF to be needled and provide ongoing functional hemodialysis at the sixth week [11,12] from the access procedure. An experienced dialysis specialist nurse determined when the fistula was ready for an attempt at cannulation and then attempted initial cannulation of the fistula; if unsuccessful, the fistula was further evaluated by the vascular surgeon at the Royal Infirmary of Edinburgh.
Statistical analysis
Statistical analysis was performed using the SPSS Statistics version 22.0 (IBM Co., Armonk, NY, USA) and Stata version 9 (StataCorp LLC, College Station, TX, USA) software. Data were expressed as the mean, standard deviation, and 95% confidence interval (CI) or as the proportion. A chi-squared test and t test were used for the comparison of independent variables between previous retrospective and prospective patients, while a P value of < 0.05 was considered to be statistically significant.
Development stage
The association between the independent factors and outcome (i.e., mature fistula) was assessed by employing univariable logistic regression. Following the univariable associations, a multivariable model was produced utilizing backward stepwise logistic regression with those variables found to be significant in the univariable regression at P < 0.25. The odds ratios (ORs) and associated 95% CIs for variables in the final model were reported. The significance level for this model was set at P < 0.05. We evaluated the calibration and discrimination performance of the model. For the calibration, the Hosmer–Lemeshow test was employed to investigate how well the predicted probabilities agreed with the observed probabilities. Discrimination, which refers to the ability of a model to distinguish between the maturation and immaturation of the AVF, was quantified using a receiver operating characteristic (ROC) curve. The ROC curve plots the sensitivity (true positive rate) against specificity (false positive rate) for consecutive “cutoffs” for the probability of an outcome [13].
Validation stage
A model precisely predicting the probabilities for patients in the retrospective data would not guarantee accurate predictions for new patients from related populations, e.g., patients treated not long ago or patients from a different center; therefore, the performance of prognostic models needed to be verified in the newly treated patient group (via external validation) [14]. Predictive logistic regression models are important tools to provide estimates of patient outcome probabilities [15]. We performed external validation on a different dataset obtained from an independent set of consecutive patients who had undergone vascular access surgery using the final development model. Validation performed on heterogeneous external datasets allows for the evaluation of the generalizability of the risk prediction tool to wider populations than originally reported. Predicted probabilities for individual patients in the validation set were calculated. Model discrimination was assessed by ROC curve analysis. An evaluation of calibration is important if model predictions are used for making clinical decisions. A calibration plot formed by the Hosmer–Lemeshow test, which illustrates how the observed and expected proportions compare, assessed the calibration of the final model for the maturation of the fistula. We plotted the observed outcome frequencies by decile of predicted probabilities.
Results
A total of 400 patients were recruited for this study, with mean ages of 60 ± 15.7 years (immature AVF group) and 60.45 ± 15.2 years (mature AVF group) (P = 0.77). The significant difference between the mature and immature AVF groups was evaluated against P < 0.05. Baseline exploration of patients’ characteristics discovered the two variables to be significantly different in both the mature and immature AVF cohorts. Patients with mature AVF were more frequently male as compared with the patients with immature AVF. The percentage of diabetics and those with PVD were higher in the immature AVF group. A significant difference (P = 0.002) was observed regarding PVD occurrence between the mature and immature AVF groups. However, no statistically significant difference (P = 0.53) was observed with respect to diabetes. The percentage of hypertensive patients, patients with a history of prior hemodialysis therapy, mean PT, and TC were also not statistically different between the mature and immature AVF groups. Overall, both cohorts’ participant characteristics were similar. Clinical and demographic characteristics of patients with mature and immature AVF are shown in Table 1.
Univariable associations
Univariable analysis found nine variables associated with the maturation of AVF: gender, side of arm, type of fistulas, PVD, diabetes, systolic BP, INR, TG, and vein size (Table 2). In addition to the above variables, a further two statistically nonsignificant variables, i.e., dialysis [16,17] (ever versus never) and eGFR [18] were added to the model due to their possible clinical association with the maturation of AVF as suggested by vascular surgeons.
Multivariable associations
Three variables were identified which were independently associated with fistula maturation using multivariable logistic regression analysis (Table 3). Males were twice as likely to undergo fistula maturation as compared with females (OR, 0.514; 95% CI, 0.308–0.857; P = 0.011) and patients who had no evidence of PVD were three times more likely to mature their fistula (OR, 3.140; 95% CI, 1.596–6.177; P < 0.001). Additionally, those with a preoperative vein diameter of greater than 2.5 mm showed a fivefold increase in fistula maturation versus those with a vein size of less than 2.5 mm (OR, 4.532; 95% CI, 2.063–9.958; P < 0.001).
The performance of the final prognostic model assessed in terms of calibration using the Hosmer–Lemeshow test (which tests the null hypothesis that the model fits the data) was not significant (P = 0.79). This suggests that there was no statistically significant difference between the predicted and observed outcomes. The area under the ROC curve for the prediction of maturation of fistula was 0.68 (95% bias-corrected CI, 0.615–0.738), which indicates fair [19] discrimination (Fig. 1).
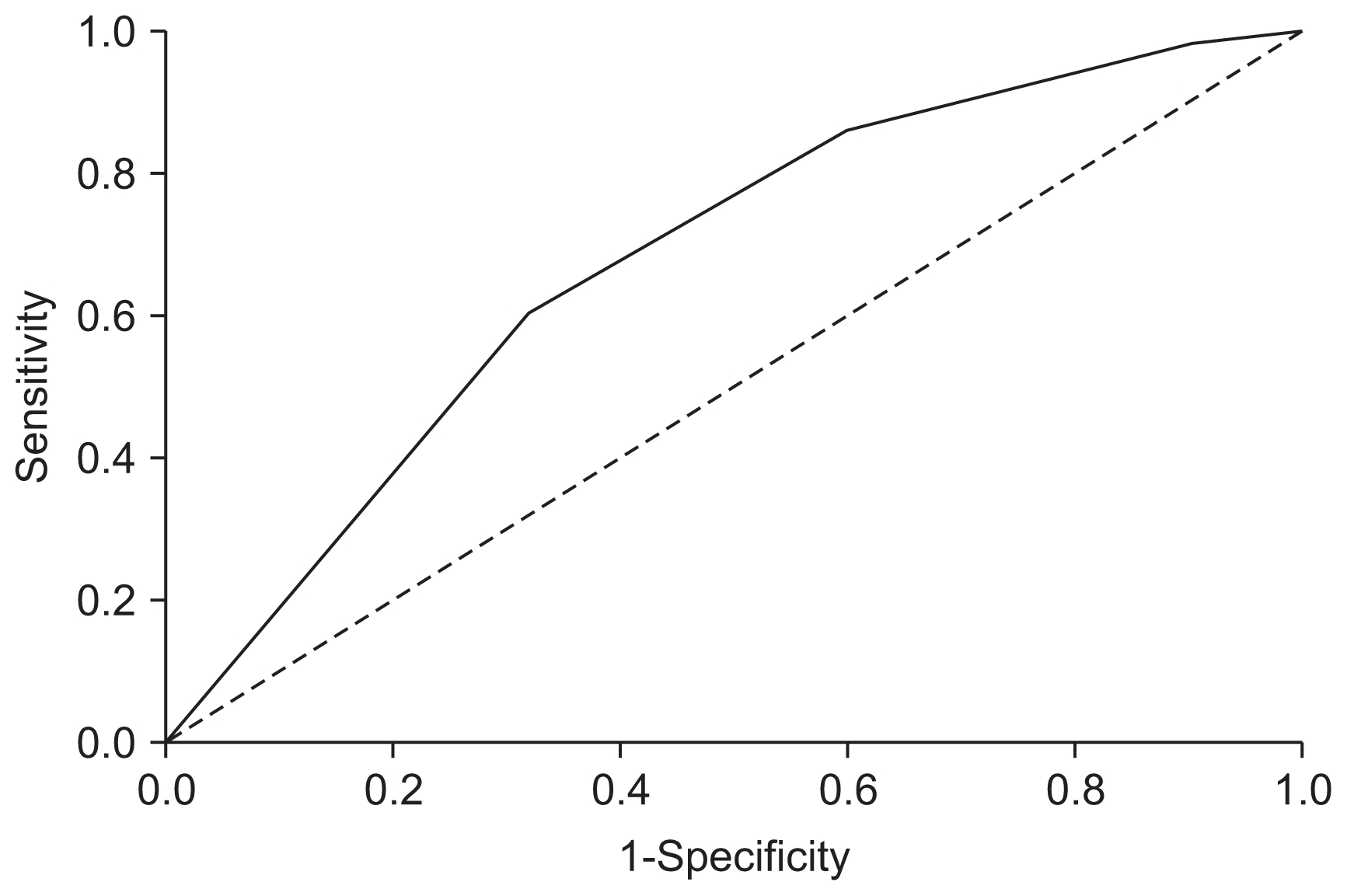
Receiver operating characteristic (ROC) curve analysis for the development of the prognostic model
It shows the ROCs of arteriovenous fistula (AVF) maturation. The area under the curve was 0.68 (95% bias-corrected confidence interval, 0.615–0.738), indicating good discriminatory ability of AVF maturation.
Model validation
We externally validated the development model, assessing aspects of discrimination and calibration. The area under the ROC curve for the prediction of the maturation of fistula was calculated using predicted probabilities and patient outcomes (mature or immature) of each patient. The ROC area was 0.59 (95% bias-corrected CI, 0.471–0.712) (Fig. 2).
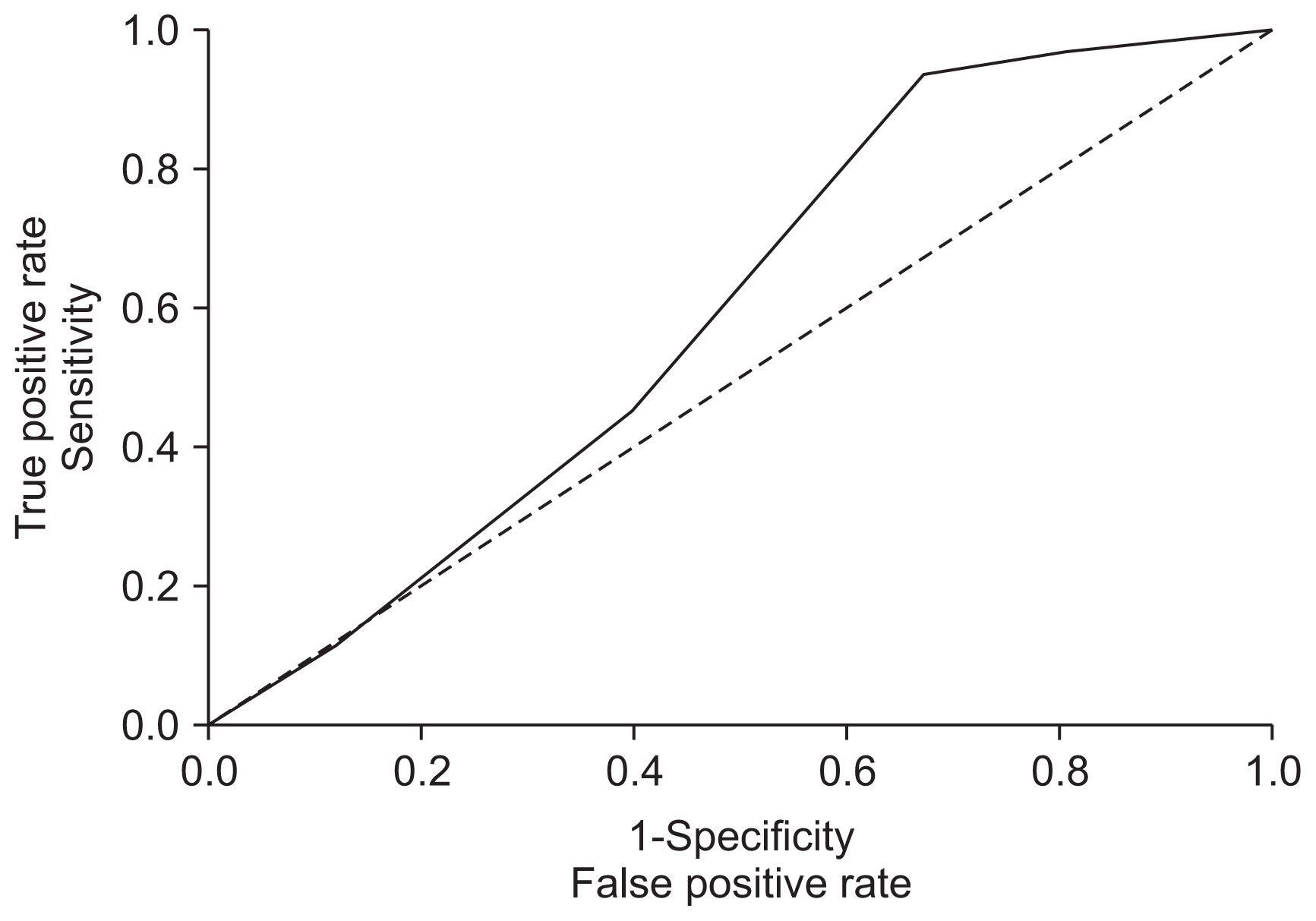
Receiver operating characteristic (ROC) curve analysis for validation of the prognostic model
It shows ROCs of arteriovenous fistula (AVF) maturation. The area under the curve was 0.591 (95% confidence interval, 0.471–0.712), indicating good discriminatory ability of AVF maturation.
A better way of assessing the fit of a logistic regression model is compare the expected and observed numbers of positives for different subgroups of the data. If the observed and expected numbers are sufficiently close to one another, then we can assume that we have an adequate model. The performance of the final prognostic model assessed in terms of calibration using Hosmer–Lemeshow test was not significant (P > 0.05). This suggests that there was no statistically significant difference between the predicted and observed outcomes.
Discussion
The long-term objective of the present research effort was to identify factors predicting fistula maturation. We hypothesized that blood and patient characteristics could be used to stratify the risk of self-report of maturation of AVF. In brief, using the development dataset of 300 subjects, we identified three variables associated with the maturation of fistula; gender, PVD, and vein size. These variables were validated by using the validation dataset of 100 subjects.
Our study results showed a gender difference, with AVF maturation rates of 60% for men and 48% for women, respectively. Miller et al [20] found that AVF maturation is more successful in men than in women. Similar results were obtained by Iyem [21]. In our study, males were twice as likely to undergo fistula maturation as compared with females, with the most rational explanation for this difference being the smaller size of vessels in women. Some studies also found female gender to be associated with immature fistula [22]. However, in contrast, there are also studies that did not find a gender difference. Başer et al [23] evaluated the creation of AVFs in 114 participants and did not observe any gender difference in terms of the maturation of AVF.
Out of 300 patients, 38% were diabetic and 16% had clinical evidence of PVD. In a number of studies, it has been reported that more than 50% of North American dialysis patients have diabetes and approximately one-third have PVD [24,25]. In our study, fistula maturation occurred at rates of 59.7% in patients with no history of PVD and 33.3% in patients with PVD. As such, this emerged as one of the predictive factors in the maturation of fistula in the multivariable analysis. Patients with no evidence of PVD were three times more likely to mature their fistula. Our data are consistent with those of other studies in which PVD was associated with AVF failure [26]. Chan et al [27] conducted a retrospective cohort analysis using 1,486 patients’ data. It was revealed that patients who suffer from PVD are more likely to experience nonmaturation of their AVFs (OR, 2.78; 95% CI, 1.01–7.63; P = 0.047). Fistula failure is consistent with the underlying need for adequate arterial vessels, which deteriorate with the normal aging process and can be damaged by concurrent disease; this finding is supported by other studies [20,28]. A recent single-center, retrospective chart review was performed by Bashar et al [29] to explore any possible association between preoperative hematological investigation and the functional maturation of AVF. A total of 86 patients (with 97 AVFs) were recruited and univariate analysis showed that female gender had a significant association with the nonmaturation of AVF. However, the study’s results did not find any association between PVD and AVF maturation. The smaller sample size as well as different study population and AVF maturation definition (functional maturation was defined as the successful use of the AVF for six consecutive sessions of hemodialysis) may have obscured this association in this investigation.
Our data are consistent with those of other studies in terms of which vein size was associated with the successful maturation of fistulas. Mendes et al [30] reported that, when the diameter of the cephalic veins exceeded 2 mm, then there was a 76% success rate of functional dialysis access, whereas, if the diameter was less than 2 mm, then there was only a 16% success rate. In the present study, a preoperative vein diameter of greater than 2.5 mm resulted in a fivefold increase in fistula maturation as compared with a vein size of less than 2.5 mm. In this regard, our data are in correlation with those of other studies in terms of which vein size is associated with successful fistula maturation [31]. The cutoff value identified by Brimble et al [32] was 2.6 mm; however, only women exhibited a substantial discrepancy between AVF success and failure with regard to vein diameter. In contrast, Wong et al [33] did not observe any discrepancies between AVF success and failure with respect to the mean vein diameter at the wrist, but indicated that AVF failure occurred in all cases where the vein diameter was less than 1.6 mm.
In research, the efficiency of a predictive model has to be tested on a new group of subjects. In the present case, the performance of the predictive model with regard to the external verification of fistulas that was constructed in the same hospital was good. The purpose of a predictive model is to assess potential risks and to manage treatment accordingly so as to ensure the success of the AVF construction procedure. This practice can help to structure the process, ensure the efficient management of resource distribution, and limit expenses. The performance of the developed model in this study was assessed by the discrimination and calibration of the model. The area under the ROC curve for a prognostic model is classically between 0.6 and 0.85 [34]. In our study, the ROC curve was primarily designed for prognostic models, rather than for diagnostic models. The ROC curve was 0.68 in the development stage and 0.59 in the validation stage, meaning that the model had a reasonable capacity to correctly distinguish between mature and immature fistulas. For clinical practice, providing insight beyond the concordance statistic has been a motivation for some recent measures, especially in the context of extension of a prediction model with additional predictive information from a biomarker or other sources [24,25]. Accuracy of the model was assessed by examining calibration [35]. To assess the validity of the predictive model developed using the development dataset, we applied the model to an independent or validation dataset composed of 100 subjects. There was excellent agreement between the predicted and observed percentages in predicting the maturation of AVF. We further note that a substantial size will be required for a validation sample to quantify validity in a reliable way, i.e., with enough power to prompt a substantial decrease in discriminative ability [36].
The present study has a number of limitations. The connection between artery diameter and blood inflow rate has been emphasized by a number of studies [31,37]. However, we did not include markers in our study due to the unavailability of retrospective data. Another limitation of this study was the considerable discrepancies in the measurements of vessel diameter in the same individual and between sonographers. Despite the effort put into monitoring every potential source of inconsistency, interobserver discrepancies in the patient sample were largely disregarded. Published studies describe hyperparathyroidism as an independent risk factor for vascular access thrombosis, probably induced by microcalcification of the vessel wall [38]; however, due to unavailability of retrospective data, we did not include these variables in the present study. Further investigations are necessary to clarify a potential relationship between hyperparathyroidism and vascular access patency. Recent studies have also suggested an important role of matrix metalloproteinase in the process of AVF maturation [39]. A promising biomarker identified in human vein tissue, matrix metalloproteinase-2, may serve to predict AVF maturation [40]. We did not look at matrix metalloproteinase as a predicative marker in the present study, however.
In summary, a preoperative, clinical prediction rule to determine fistulae that are likely to mature was created based on single-center analyzable datasets in AVF creation. Gender, PVD, and vein size are useful predictors of AVF maturation. The developed model was validated using the validation dataset. The clinical utility of these risk categories in the maturation of AVF requires further clinical evaluation in longer follow-up periods with the inclusion of additional variables such as diameter and blood flow of arteries. This could be done in the context of larger multicenter trials where treatment, follow-up, and endpoints are predefined and measured according to identical criteria in all patients.
Acknowledgments
We wish to thank Rona Lochiel, a vascular access nurse specialist at the Royal Infirmary of Edinburgh for her assistance in the recruitment and follow-up of the study participants. We also thank statistician Catriona Graham (Wellcome Trust Clinical Research Facility, University of Edinburgh) for her advice in the data analysis.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.


