| Kidney Res Clin Pract > Volume 37(3); 2018 > Article |
|
Abstract
Background
This study was undertaken to explore the effects of aging on the kidneys in mouse models of diabetes and chronic kidney disease (CKD), and to compare the expression of two isoforms of matrix metalloproteinase-2 (MMP-2)–secretory full-length MMP-2 and intracellular N-terminal truncated MMP-2 (NTT-MMP-2)–in these models.
Methods
Two experimental ICR mouse models were used: a streptozotocin (STZ)-induced type 1 diabetes mellitus model and a 5/6 nephrectomized (5/6Nx) CKD model. The abundance of each isoform of MMP-2 was determined by quantitative polymerase chain reaction (qPCR), and functional analyses were conducted. Moreover, the protein levels of the two MMP-2 isoforms were determined semi-quantitatively by immunohistochemical staining, and their association with tissue damage was assessed.
Results
Both isoforms of MMP-2 were upregulated in the kidney tissues of STZ-induced diabetic mice and 5/6Nx mice, irrespective of age. Characteristically, NTT-MMP-2 protein expression was elevated in old control mice, in line with the qPCR results. NTT-MMP-2 expression was limited to the renal cortex, and to the tubulointerstitial area rather than the glomerular area. In terms of tissue damage, tubulointerstitial fibrosis was more severe in old 5/6Nx mice than in their young counterparts, whereas glomerulosclerosis was comparable in old and young 5/6Nx mice.
Ageing is related to organ dysfunction and can disrupt homeostasis in the human body. However, the precise mechanisms of ageing are not known, and intensive work continues on this topic [1,2]. Many structural and functional changes occur in the kidneys during ageing, such as glomerulosclerosis, tubular atrophy, interstitial fibrosis, atherosclerosis and hypertrophy. In addition, ageing can increase renal susceptibility to injury or damage, such as ischemia-reperfusion, toxin-mediated and metabolic injuries, leading to pathologic processes and reduced regenerative potential. In short, kidney ageing is a potent risk factor for renal disease [3–7].
Ageing and chronic kidney disease (CKD) are highly related to oxidative stress. Because transport activities are conducted mainly in the proximal tubules, mitochondria are abundant and antioxidant enzyme expression is elevated in this region. However, ageing diminishes antioxidant enzyme activity and could augment oxidative injury in the renal tubules [8,9].
Diabetes mellitus is the most common etiology of CKD and is associated with cellular senescence in several tissues, including the kidneys [3,10,11]. Senescence of the proximal tubular cells can potentiate the renin-angiotensin-aldosterone system [12].
Our laboratory previously reported that the full-length, secreted isoform of matrix metalloproteinase-2 (FL-MMP-2) was related to progressive renal injury, based on observations in a transgenic murine model of FL-MMP-2 specific to renal tubular epithelial cells [13]. More recently, a novel isoform of matrix metalloproteinase-2 (MMP-2), intracellular 65-kd MMP-2, was detected in mitochondrial fractions from several murine models, including the FL-MMP-2 transgenics, ageing mice and a model of accelerated atherosclerosis [14]. This novel isoform, N-terminal truncated MMP-2 (NTT-MMP-2), is characterized by N-terminal truncation and intracellular localization, and is synthesized by translation from M77 in the second exon. We previously demonstrated that NTT-MMP-2 was upregulated by oxidative stimuli such as high glucose, and that its expression was enhanced in an experimental diabetic animal model and in human diabetic nephropathy [15]. Currently, NTT-MMP-2 is thought to be related to tubular epithelial cell-regulated necrosis, especially in renal proximal tubular cell-specific NTT-MMP-2 transgenic mice [16].
The present study was undertaken to explore the effects of ageing on the kidneys in mouse models of diabetes and CKD, and to compare the expression of the FL-MMP-2 and NTT- MMP-2 in these models.
The animal protocol (2016-088, 2016-089) used in this study was reviewed and approved by the Pusan National University–Institutional Animal Care and Use Committee with respect to procedural ethicality and scientific care. The murine models used were a streptozotocin (STZ)-induced model of type 1 diabetes mellitus and a kidney remnant model (produced by 5/6 nephrectomy [5/6Nx]) of CKD.
The diabetic model was induced through five daily intraperitoneal injections of STZ (40 mg/kg in citrate buffer, pH 4.5; Sigma-Aldrich, St. Louis, MO, USA) to 8-week-old ICR mice (young mice) and 14-month-old ICR mice (old mice). Control mice received citrate buffer alone. One week after the completion of the STZ treatment (1-week post-STZ), the glucose levels in tail vein blood were measured with a blood glucometer. Body weights were measured weekly and blood glucose concentrations were monitored in the first 2 weeks and every four weeks thereafter to confirm hyperglycemia. STZ-induced diabetic mice were euthanized under isoflurane anesthesia 12 weeks after the completion of the STZ injections. The kidneys were perfused with 4°C phosphate-buffered saline (PBS), excised and fixed in 10% neutral formalin for light microscopic analysis and immunohistochemistry (IHC); the remaining portions were used for quantitative polymerase chain reaction (qPCR) analyses. To examine the effects of ageing on diabetes, we allocated five animals to each of the following four groups: Group I, young control group (Y-C); Group II, old control group (O-C); Group III, young diabetes mellitus group (Y-DM); and Group IV, old diabetes mellitus group (O-DM).
The murine model of CKD was produced by 5/6Nx of ICR mice under isoflurane anesthesia. First, a partial nephrectomy of the upper and lower poles of the left kidney was carefully performed so that the ureteral and adrenal gland would not be damaged, and the resected renal parenchyma was then weighed. Subsequent bleeding was controlled with an absorbable collagen hemostat (Kyeron, Enschede, Netherlands). One week later, a right total nephrectomy was performed and the entire right kidney was weighed. All the included CKD animals had a left excised kidney tissue/right total kidney weight ratio greater than 0.5.
The nephrectomy procedure was conducted on young and old ICR mice as with the DM groups. Animals were euthanized under isoflurane anesthesia at 4 weeks post-5/6Nx. The kidneys were perfused with 4°C PBS, excised and fixed in 10% neutral formalin for light microscopic analysis and IHC, and the remaining portions were subjected to qPCR or western blotting. To determine the effects of ageing on animals with CKD, we allocated five mice to each of the following groups: Group I, young control group (Y-C); Group II, old control group (O-C); Group III, young 5/6Nx group (Y-5/6Nx); and Group IV, old 5/6Nx group (O-5/6Nx). The control groups (Groups I and II) were the same ones used in the above diabetic experiment.
Serum creatinine levels and urinary albumin to creatinine ratios were measured as functional parameters in both murine models by means of enzyme-linked immunosorbent assays (ELISA) (#80350, mouse creatinine kit; Crystal Chemistry, Downers Grove, IL, USA and AKRAL-121 mouse albumin ELISA kit; Shibayagi Co., Ltd., Shibukawa, Japan, respectively).
FL-MMP-2 and NTT-MMP-2 messenger RNA (mRNA) levels in kidney tissues were determined by qPCR. Total RNA was isolated with TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. In brief, cDNA was synthesized from 2 μg of total RNA by means of oligo-dT primers and M-MLV RTase (Promega, Madison, WI, USA) for 1 hour at 42°C; the primers used for real-time PCR are summarized in Table 1. Amplification was conducted for 40 cycles (95°C for 15 seconds; 60°C for 45 seconds; and 72°C for 1 minute) with Fast Start Universal SYBR Green Master Mix (Rox dye; Roche, Basel, Switzerland) on an ABI 7500 Real-time PCR System (Applied Biosystems, Foster City, CA, USA). As a housekeeping internal control, β-actin was quantified in parallel with the target genes, and all products were verified by melting curve analysis (95°C for 15 seconds, 60°C for 15 seconds, 95°C for 15 seconds). Genes were normalized and fold-changes were calculated by the 2−ΔΔCT method.
Immediately after collection, tissues were fixed in 10% formalin, paraffin-processed and embedded. IHC was performed on 3-μm-thick formalin-fixed paraffin-embedded sections after the sections had been deparaffinized and rehydrated through an ethanol series. For FL-MMP-2 immunostaining, sections were incubated for 30 minutes with a prediluted monoclonal anti-mouse antibody against the N-terminal sequence of the FL-MMP-2 protein (MA5-13590; Thermo Fisher Scientific, Rockford, IL, USA), and were then incubated for 30 minutes with biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA). For NTT-MMP-2 isoform immunostaining, sections were incubated overnight at 4°C with the isoform-specific antibody at 5 μg/mL. After each primary antibody treatment, the sections were incubated for 30 minutes with a secondary biotinylated antibody (Vector), and then were incubated with Vectastain ABC complex (Vector). In the case of STZ kidneys, immunohistochemical development was performed with VIP peroxidase substrate (Vector) and counterstaining was performed with methyl green. For 5/6Nx kidneys, immunohistochemical development was performed with 3, 3′-diaminobenzidine, counterstaining was performed with hematoxylin, and the staining intensities were assessed semi-quantitatively by the following grading system: grade 0, negative; grade 1, weak patchy staining; grade 2, weak diffuse or dense patchy staining; grade 3, dense diffuse staining.
Statistical analyses were performed with GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). The Kruskal–Wallis test with Dunn’s multiple comparison or the Mann–Whitney U test was used to compare experimental groups as appropriate. Statistical significance was accepted for P values < 0.05. The results are presented as the mean ± standard deviation.
The serum creatinine levels and urinary albumin to creatinine ratios did not differ significantly among the four groups of mice in the diabetes experiment (Fig. 1A, B). The qPCR demonstrated that FL-MMP-2 and NTT-MMP-2 transcripts were more abundant in old control mice than in young control mice (Fig. 1C, D). At 12 weeks post-STZ, FL-MMP-2 transcript levels were 5.87 ± 2.92- and 5.42 ± 3.29-fold higher in young and old diabetic mice, respectively, than in young controls (P = 0.001), but no significant difference was observed between young and old control mice. NTT-MMP-2 transcript levels were 12.79 ± 6.71- and 20.81 ± 26.94-fold greater in young and old diabetic mice, respectively, than in young controls (P < 0.001). Interestingly, unlike FL-MMP-2 levels, NTT-MMP-2 mRNA levels were 1.66 ± 1.72-fold higher in old controls than in young controls.
IHC staining of renal cross-sections revealed that FL-MMP-2 was expressed faintly in the renal cortices and medullae of young and old mice, and was expressed at higher levels in diabetic mice than in young controls (Fig. 2A–D), consistent with our qPCR results. NTT-MMP-2 was barely detectable in the cortices of old control kidneys, was not detected in young control kidneys, and was intensely expressed in young and old diabetic kidneys (Fig. 2E–H). These patterns of the two isoforms of MMP-2 are summarized semi-quantitatively in Fig. 2I, J. NTT-MMP-2 expression was limited to the cortices, whereas FL-MMP-2 expression was not.
Fig. 2K provides a detailed summary of periodic acid-Schiff (PAS), Masson’s trichrome (MT) and IHC staining by high power field. Some interstitial fibrosis was observed in old control mice and diabetic mice by MT staining, but no fibrosis was observed in the cortices of young controls. Tubular injuries including tubular cast and dilatation were observed in young diabetic mice, but were aggravated in old diabetic mice, as determined by PAS staining. FL-MMP-2 expression was stronger in diabetic mice than in controls, irrespective of age, and NTT-MMP-2 expression was stronger in diabetic mice than in young controls. Interestingly, in old control mice, NTT-MMP-2 was expressed focally and at an obviously higher intensity than in young controls, in line with our qPCR results.
Serum creatinine levels were higher in 5/6Nx mice (irrespective of age) than in young controls (Fig. 1D: 3.21 ± 1.16 in Y-5/6Nx and 1.89 ± 0.99 in O-5/6Nx vs. 0.94±0.40 μg/mL in Y-C). Consistent with the serum creatinine levels, the urinary albumin to creatinine ratios were also higher in 5/6Nx mice than in young controls (Fig. 1F: 2,663 ± 686 in Y-5/6Nx and 2,249 ± 1,169 in O-5/6Nx vs. 88.84 ± 45.39 μg/mg creatinine in Y-C). FL-MMP-2 mRNA levels were 3.49 ± 1.49-fold higher in old 5/6Nx mice than in the other three groups at 12 weeks post-STZ (P = 0.003); however, no significant difference was observed among the young controls, old controls and young 5/6Nx mice. NTT-MMP-2 transcript levels were significantly higher in young and old 5/6Nx mice than in young controls (6.63 ± 0.14- and 2.93 ± 2.21-fold, respectively; P < 0.005) (Fig. 1G, H).
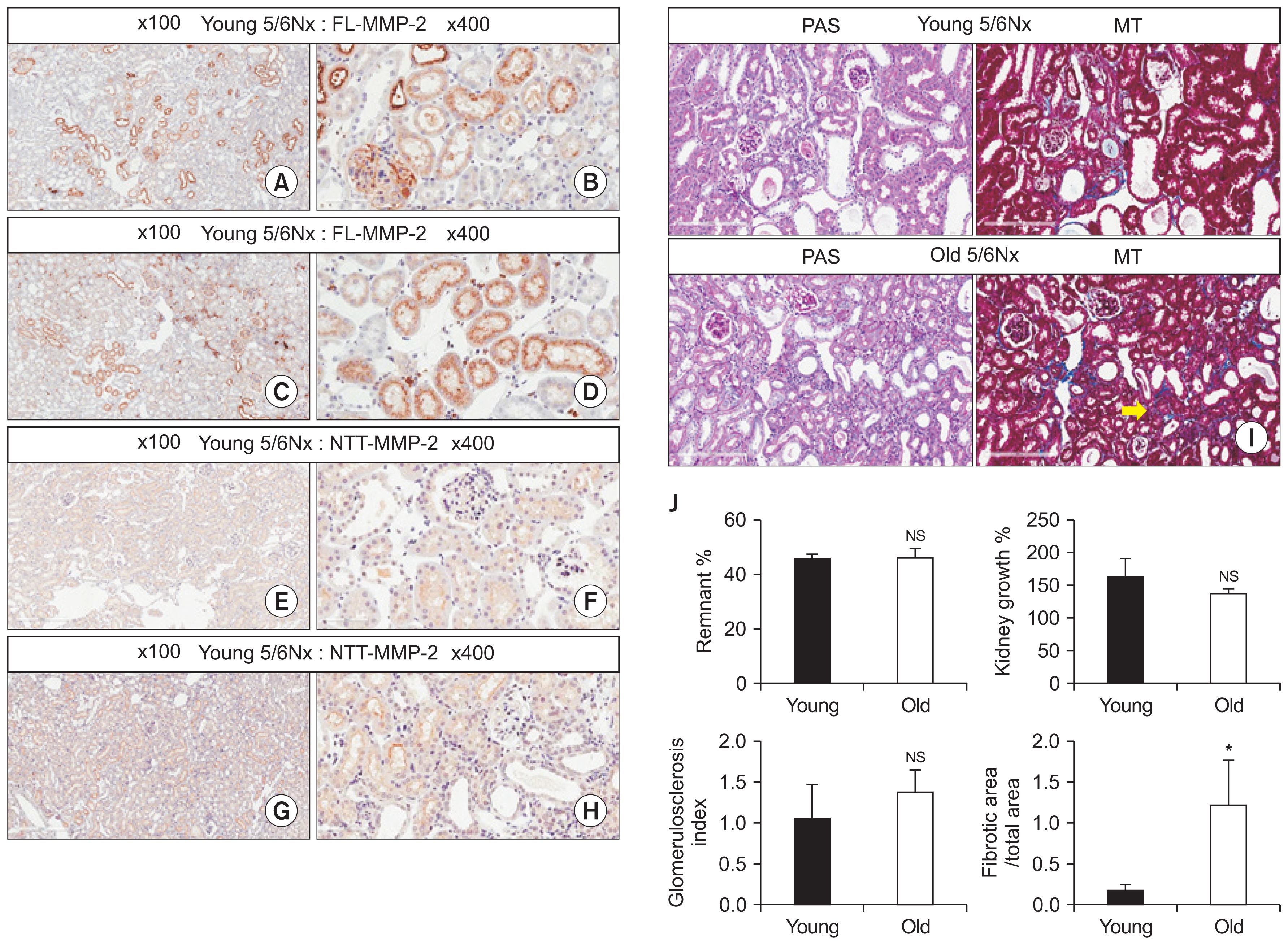
Fig. 3A–H summarize the results of IHC staining for these two isoforms of MMP-2 in the renal cortices, and demonstrates the effects of ageing in our CKD model. FL-MMP-2 levels were comparable in young and old 5/6Nx mice, and this isoform was found to be localized to the glomeruli and renal tubules (Fig. 3A–D). NTT-MMP-2 levels were comparable in young and old 5/6Nx mice, but staining was mainly limited to the renal tubules (Fig. 3E–H). Although the remnant kidney growth rates were comparable in the two groups, tubulointerstitial fibrosis was more severe in old 5/6Nx mice than in their young counterparts, while the severity of glomerulosclerosis was similar in young and old 5/6Nx mice in terms of tissue damage (Fig. 3I, J). These findings suggest that ageing aggravates tubulointerstitial fibrosis in the setting of CKD.
The principal findings of this study are that two isoforms of MMP-2 (FL-MMP-2 and NTT-MMP-2) were induced in the kidneys of old and young murine models of type 1 diabetes mellitus and CKD, and that NTT-MMP-2 transcript levels increased with ageing, regardless of the presence of these disease conditions. Furthermore, ageing was associated with more severe tubulointerstitial fibrosis in 5/6Nx mice.
Previously, we found that a novel MMP-2 signal in mitochondrial preparations from aged murine hearts was induced by oxidative stress in H9C2 cells [14], and we named this isoform NTT-MMP-2. This truncated isoform was found to be generated by the activation of an alternative promoter in the distal first intron of the MMP-2 gene [14]. Oxidative stress is a major culprit in diabetic nephropathy, and the two MMP-2 isoforms are induced by STZ (type 1 diabetic murine model) and have been shown to be related to renal tubular injury [15,17]. Hyperglycemia can induce oxidative stress, and was found to result in the earlier induction of FL-MMP-2 and NTT-MMP-2 in an in-vivo study conducted with a proximal tubular cell line, HK2 cells [15]. Moreover, many factors, including uremic toxins, are known to activate oxidative stress by initiating the abundant production of reactive oxygen species, even during early CKD [18,19].
In terms of ageing, cellular senescence is related to oxidative stress and mitochondrial damage, and ageing increases the renal sensitivity to injury and adversely affects renal recovery after injury [20]. Interestingly, we found that NTT-MMP-2 was induced in old control mice, indicating that ageing per se might increase the risk of damage caused by injurious stimuli. In a previous study in proximal tubular cell-specific NTT-MMP-2 transgenic mice, we observed that NTT-MMP-2 induced tubular regulated necrosis in a murine model of ischemia-reperfusion injury, resulting in inflammation and fibrosis in kidney tissues [16]. Consistent with these results, NTT-MMP-2 has been associated with nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB), and the inhibition of NF-κB was found to downregulate NTT-MMP-2 in HK2 cells [15]. In the present study, we observed no difference between young and old diabetic mice, possibly because STZ-induced renal injury is mild, even when blood glucose levels are extremely high. However, renal tubulointerstitial fibrosis was more severe in old 5/6Nx mice than in their young counterparts, suggesting that NTT-MMP-2 upregulation was related to more severe renal damage in the remnant murine model, and that ageing contributed to these detrimental effects. Furthermore, unlike FL-MMP-2 expression, NTT-MMP-2 expression was confined to the renal cortices, supporting the role of NTT-MMP-2 in the tubulointerstitial pathologic changes observed in CKD.
Although no data are available on the roles of the FL-MMP-2 and NTT-MMP-2 isoforms in CKD, we found that both were expressed in our 5/6Nx remnant murine model of CKD and in our type 1 diabetes model. These findings may provide a basis for further studies aimed at delineating the injurious mechanisms in CKD of various etiologies.
In conclusion, we found that the intracellular isoform of MMP-2, NTT-MMP-2, was induced by ageing, irrespective of STZ-induced diabetes mellitus or 5/6 nephrectomy-induced CKD in our murine models, and that this induction might be related to the development of tubulointerstitial fibrosis in CKD. Future studies are required to identify the molecular mode of action of NTT-MMP-2 and to devise drugs that target biomolecules involved in the development of chronic kidney disease.
Acknowledgments
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2016R1A2B4008243). DHL was supported by the Department of Veterans Affairs Merit Review Award I-1BX000593 and the National Institute of Diabetes, Digestive and Kidney Disease grant RO1DK39776.
Figure 1
Renal function and qPCR measurement of FL-MMP-2 and NTT-MMP-2 transcript levels in streptozotocin-induced diabetic mice and 5/6 nephrectomized (5/6Nx) mice
The serum creatinine levels and urinary albumin to creatinine ratios were evaluated in the four groups of mice in each experiment, and qPCR was performed on transcripts isolated from whole kidneys in each group. (A–D) Group I, young control group (Y-C); Group II, old control group (O-C); Group III, young diabetes mellitus group (Y-DM); Group IV, old diabetes mellitus group (O-DM). (E–H) Group I, young control group (Y-C); Group II, old control group (O-C); Group III, young 5/6 nephrectomy group (Y-5/6Nx); Group IV, old 5/6 nephrectomy group (O-5/6Nx).
n = 5 for each group; *P < 0.05 compared with young controls.
MMP-2, matrix metalloproteinase-2; FL-MMP-2, full-length MMP-2; NTT-MMP-2, N-terminal truncated MMP-2; qPCR, quantitative polymerase chain reaction.
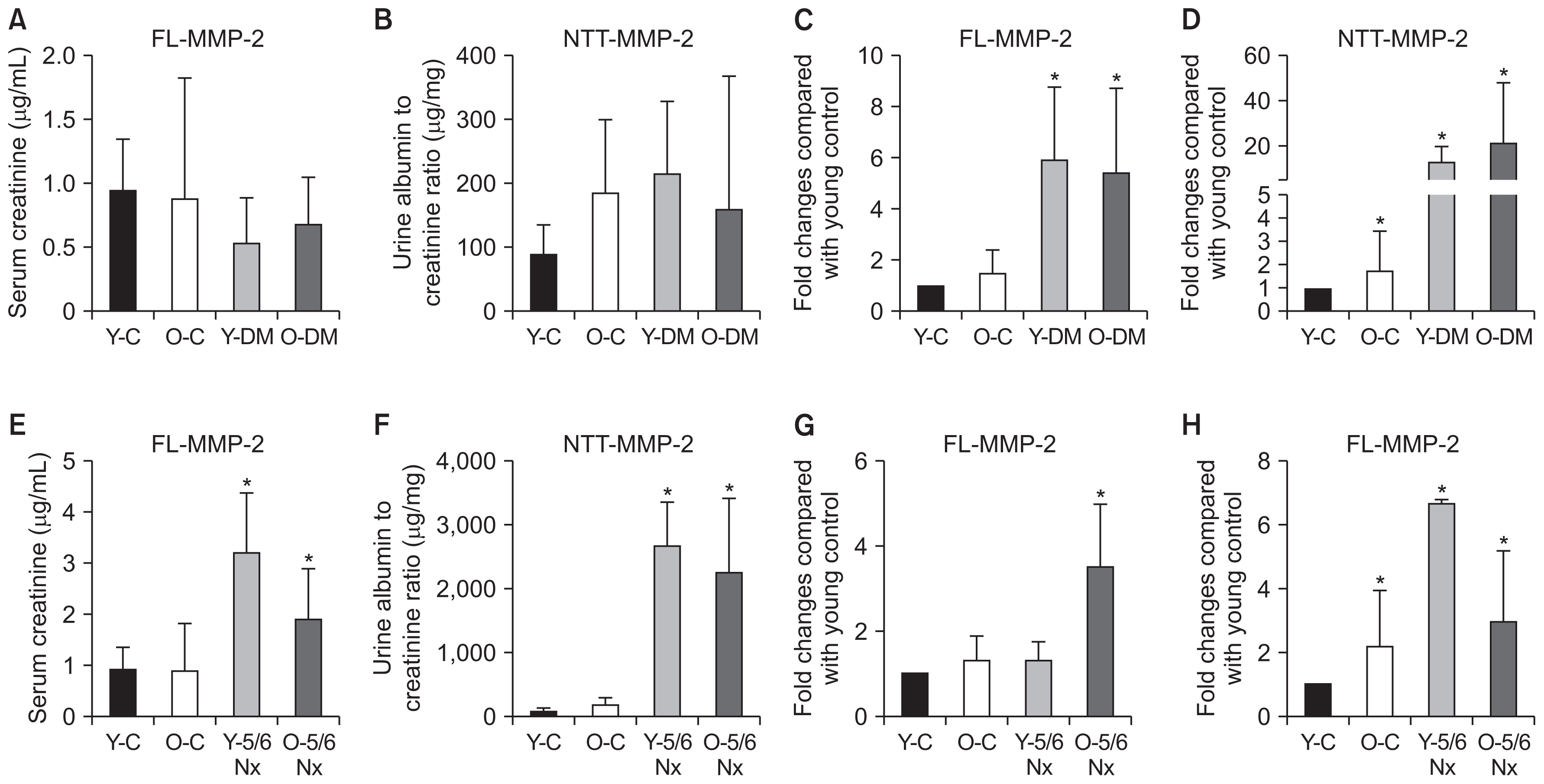
Figure 2
Histological assessment of kidneys in the streptozotocin model of type 1 diabetes mellitus
(A–J) Immunohistochemical staining for FL-MMP-2 and NTT-MMP-2: (A–D) FL-MMP-2 was faintly detectable in young and old control mice, and its expression increased with the induction of diabetes (×10); (E–H) NTT-MMP-2 was faintly detectable in old control mice, and its expression increased with the induction of diabetes; (I, J) semi-quantitative scoring of IHC staining (n = 5, *P < 0.05 compared with young controls). (K) Detailed microscopic findings (×200). The first row presents that interstitial fibrosis was observed in old control mice and diabetic mice in Masson’s trichrome (MT) staining. The second row presents that tubular injury was noted in old mice. In young diabetic mice, tubular injuries including tubular cast (black arrow) and dilatation (yellow arrow) were observed, and these findings were aggravated in old diabetic mice in periodic acid-Schiff (PAS) staining. The third row presents that FL-MMP-2 expression was stronger in diabetic mice than in control mice, irrespective of ageing status. The last row presents that NTT-MMP-2 expression was stronger in diabetic mice than in young control mice. Interestingly, in old control mice, NTT-MMP-2 was expressed focally and at a higher intensity than in young control mice (black arrow).
MMP-2, matrix metalloproteinase-2; FL-MMP-2, full-length MMP-2; NTT-MMP-2, N-terminal truncated MMP-2.

Figure 3
Histological assessment of kidneys in 5/6 nephrectomized (5/6Nx) mice
(A–H) Immunohistochemical staining of the kidneys for FL-MMP-2 and NTT-MMP-2 in the 5/6Nx mouse model: (A–D) The levels of FL-MMP-2 were comparable in young and old 5/6Nx mice. There were stained areas in the glomeruli and renal tubules (×100 and ×400); (E–H) The levels of NTT-MMP-2 were comparable in young and old 5/6Nx mice. The principal stained areas were in the renal tubules (×100 and ×400). (I) Glomerular and tubulointerstitial damage in young and old 5/6Nx mice (×400). Upper panel: periodic acid-Schiff (PAS) and Masson’s trichrome (MT) staining in young 5/6Nx mice. There were segmental sclerotic areas in some glomeruli, and there was inflammation with fibrosis and tubular injury in the tubulointerstitial area. Lower panel: PAS and MT staining in old 5/6Nx mice. There were segmental sclerotic areas in the glomeruli, as in the young 5/6Nx model. However, tubulointerstitial fibrosis was more prominent in old 5/6Nx mice (yellow arrow). (J) Comparison of the characteristics between young and old 5/6Nx mice. There were no differences in the remnant percent of the renal parenchyma, the percent growth rate for 1 month and the glomerulosclerosis index between the groups. The only significant finding was that old 5/6Nx mice had more fibrotic areas in the tubulointerstitial area than young 5/6Nx mice (n = 5, *P < 0.05).
MMP-2, matrix metalloproteinase-2; FL-MMP-2, full-length MMP-2; NTT-MMP-2, N-terminal truncated MMP-2.
References
2. Rose MR. Evolutionary Biology of Aging. New York: Oxford University Press; 1991.
3. Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal ageing and disease. Nat Rev Nephrol 13:77–89. 2017;


4. Tan JC, Busque S, Workeneh B, et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int 78:686–692. 2010;



5. Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 152:561–567. 2010;



6. Elsherbiny HE, Alexander MP, Kremers WK, et al. Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol 9:1892–1902. 2014;



7. Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One 8:e704642013;



8. Thiab NR, King N, Jones GL. Effect of ageing and oxidative stress on antioxidant enzyme activity in different regions of the rat kidney. Mol Cell Biochem 408:253–260. 2015;


9. Chandran G, Sirajudeen KN, Yusoff NS, Swamy M, Samarendra MS. Effect of the antihypertensive drug enalapril on oxidative stress markers and antioxidant enzymes in kidney of spontaneously hypertensive rat. Oxid Med Cell Longev 2014:6085122014;



10. Jin DC, Yun SR, Lee SW, Han SW, Kim W, Park J. Current characteristics of dialysis therapy in Korea: 2015 registry data focusing on elderly patients. Kidney Res Clin Pract 35:204–211. 2016;



11. Verzola D, Gandolfo MT, Gaetani G, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol 295:F1563–F1573. 2008;


12. Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530:184–189. 2016;



13. Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J 20:1898–1900. 2006;


14. Lovett DH, Mahimkar R, Raffai RL, et al. A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity. PLoS One 7:e341772012;



15. Kim SS, Shin N, Bae SS. Enhanced expression of two discrete isoforms of matrix metalloproteinase-2 in experimental and human diabetic nephropathy. PLoS One 12:e01716252017;



16. Ceron CS, Baligand C, Joshi S, et al. An intracellular matrix metalloproteinase-2 isoform induces tubular regulated necrosis: implications for acute kidney injury. Am J Physiol Renal Physiol 312:F1166–F1183. 2017;



17. Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57:1446–1454. 2008;


18. Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65:1009–1016. 2004;





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















