| Kidney Res Clin Pract > Volume 36(2); 2017 > Article |
|
Abstract
Background
Salvage of a thrombosed arteriovenous fistula (AVF) by secondary fistula conversion may be more effective than a conventional endovascular procedure for forearm fistula thrombosis. Surgical access procedures are an undeveloped area in interventional nephrology compared to endovascular procedures. Herein, the author report the results of surgical salvage of thrombosed AVFs by interventional nephrologists.
Methods
The author retrospectively analyzed 52 surgical salvage procedures for AVF thrombosis (radiocephalic fistula = 44 cases, brachiocephalic fistula = 8 cases) that were performed by interventional nephrologist between March 2007 and January 2016.
Results
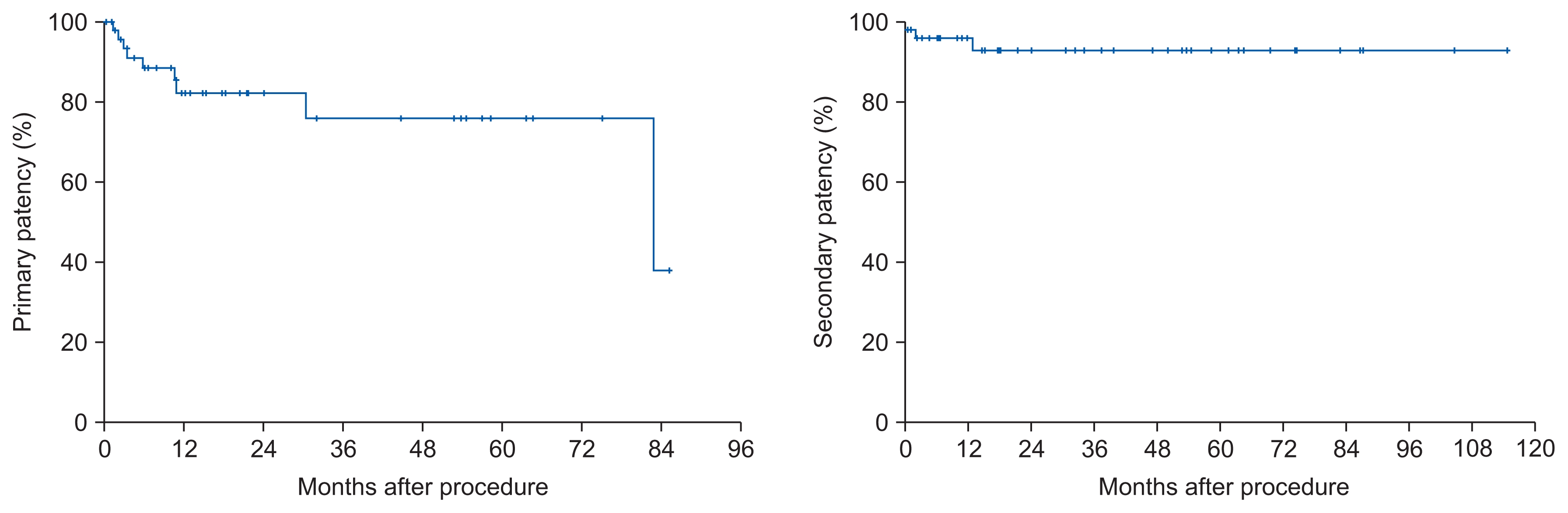
Secondary fistula formation using the proximal vein was performed for 46 cases (88.5%); outflow rerouting was performed for two cephalic-arch stenosis cases (3.9%), simple thrombectomy was performed for two cases (3.9%), and a graft interposition was performed for two cases (3.9%). Technical success after the surgical procedures was achieved in 51 cases (98.1%), and 39 AVFs (75.0%) were prepared for immediate puncturing without catheter insertion. The primary and secondary patency rates for AVF at 6, 12, 18, and 24 months were 88.5%, 83.2%, 83.2%, and 83.2% and 96.0%, 96.0%, 93.2%, and 93.2%, respectively. The re-intervention rate was 0.27 ┬▒ 0.92/patient/ year.
Salvage of a thrombosed arteriovenous fistula (AVF) is at least equally important as the timely placement of access at the beginning of hemodialysis therapy [1]. Many methods, such as percutaneous thrombectomy [1], surgery [2] or hybrid procedures [3], have been performed in accordance with local experience at various centers with varying success rates. Success rates depend on the local experience of the surgical team, and evidence suggests that surgeries that use a mature patent outflow vein are superior to percutaneous thrombectomy for treating AVF thrombosis caused by peri-anastomotic stenosis in forearm fistulas [4]. Interventional nephrology is a rising subspecialty in South Korea. However, compared to percutaneous procedures, surgical access management is developing more slowly among interventional nephrologists. Surgical salvage can have high long-term success rates if practiced without delay and may be more cost-effective than endovascular procedures [5ŌĆō7]. The nephrologist who performed the surgeries that the author evaluated herein has been involved in developing surgical salvage techniques for AVF at the Department of Nephrology, since 2006. Herein, the author report results of clinical experience from surgical salvage procedures for thrombosed AVFs performed by the above-mentioned interventional nephrologist.
Surgical salvage cases of 52 thrombosed AVFs performed between March 2007 and January 2016 were retrospectively analyzed. Physical (length and compressibility of thrombosed AVF) and ultrasound examinations (diameter of feeding artery, patency and diameter of anastomosis, presence of eventual low blood flow through the AV fistula, length of thrombus, compressibility of AVF vein, and site of initiating stenotic lesion) by a nephrologist preceded all surgical procedures. Fistula stenotic lesions that cause thrombosis were classified as 3 types stenosis [5]. Type-1 stenosis is a juxta-anastomotic stenosis. Type-2 stenosis is needling-segment stenosis or stenosis that occurs at the outflow vein. Type-3 stenosis is a junctional area stenosis, such as a cephalic-arch stenosis.
Most surgical procedures were outpatient procedures, performed with local anesthesia (2% lidocaine) using microsurgical instruments and magnifying glasses (2.5├Ś magnification) by the same interventional nephrologist who performed the ultrasound; the nephrologist was specialized in and experienced with vascular-access procedures. A Fogarty catheter was used to remove the thrombus. Reanastomosis was sutured with a 6.0 or (usually) 7.0 polypropylene suture. The wound was sewn in two layers. If the vein near the anastomosis was narrowed, a new arteriovenous anastomosis was created a few centimeters (usually 3ŌĆō5 cm) proximal to the original anastomosis. The general principal was to create a new anastomosis as distal from the fistula as possible. A secondary fistula was created at elbow level in a few cases of radiocephalic fistula in which the forearm outflow veins were diffusely stenosed but the upper-arm vein was intact. In cephalic-arch stenosis-related thrombosis cases, the cephalic vein was transected and transposed to the adequate veins (diameters greater than 4 mm in ultrasound) such as basilic vein, brachial vein or axillary vein for outflow relocation. In a few cases, a simple thrombectomy without reanastomosis was carried out with a simple Fogarty balloon catheter and by manually squeezing the AVF vein at the proximal end toward its distal end in order to promote expulsion of the thrombus. The author performed sono-guided angioplasty to treat stenosis that was detected intraoperatively because this policy makes surgery more simple and economic. The surgical strategy used for each patient was determined according to clinical and ultra-sonographic findings and was modified during surgery if necessary. Following surgery, patients usually remained under medical supervision for two hours and then were discharged from the emergency department. Technical success was defined as the successful restoration of AVF flow with uneventful dialysis after the procedure. Primary patency was calculated from the date of the initial salvage procedure to the next subsequent access intervention. Secondary patency was calculated from the date of the initial salvage procedure to the follow up end or permanent access loss. Fistula follow-up was censored for patient death, transplant, and change of dialysis modality.
Demographic and baseline clinical characteristics are reported as means and standard deviations, median with ranges or proportions, as appropriate. Baseline characteristics were compared using t-test and Žć2 test as appropriate. Primary and secondary patency values were derived with the Kaplan-Meyer survival curve. All analyses were performed using the Statistical Package for the Social Sciences for Windows (IBM SPSS Statistics, version 22.0; IBM Co., Armonk, NY, USA).
The author retrospectively analyzed surgical salvage procedures for a total of 52 autogeneous AVF thrombosis cases (radiocephalic fistula [RCF], 44 cases; brachiocephalic fistula [BCF], 8 cases) that were performed between March 2007 and January 2016. This study included 31 men and 21 women, aged 16ŌĆō89 years (average, 61.7 ┬▒ 15.8 years). Diabetes mellitus was present in 51.9% (27/52) of the patients, and the AVF median vintage at thrombosis was 14.9 (0.5ŌĆō128.5) months. The time from AVF thrombosis to surgical salvage was 1.7 ┬▒ 0.8 days (Table 1). Twenty (38.5%) patients had a history of percutaneous intervention of the same access before this procedure. RCF thrombosis cases included 35 pathologic stenotic lesions located in the juxta-anastomotic area, and thrombosis was confined to the juxta-anastomotic area (type-1 stenosis). Stenosis was located in the outflow cephalic vein and thrombosis was located in mid-forearm veins in nine cases (type-2 stenosis). Treatment of type-1 stenosis in RCF cases included proximal re-anastomosis procedures, which were performed in 30 of 35 cases (85.7%), elbow-area fistula formation was performed in 4 of 35 cases (11.4%), and polytetrafluoroethylene (PTFE) jump-graft procedures were performed in 1 of 35 cases (2.9%). Treatment for type-2 stenosis in RCF included reanastomosis of the proximal forearm veins in 2 of 9 cases (22.2%); elbow-area fistula formation in 6 of 9 cases (66.7%); and a simple Fogarty thrombectomy in 1 of 9 cases (11.1%) (Table 2). Only 8 cases of surgical reconstruction were performed in upper arm, of which 4 were type-1 stenosis, 1 was a type-2 stenosis, and 3 were cephalic-arch (type-3) stenosis. Four type-1 stenosis cases were treated by proximal reanastomosis. Fogarty thrombectomy and ultrasound guided angioplasty were performed for one case of outflow vein stenosis (type-2). Three cephalic-arch stenosis cases were treated by cephalic-vein-to-brachial-vein anastomosis (autogenous, 2 cases; PTFE, 1 case) (Table 2).
Overall immediate technical success was achieved in 51 cases (98.1%), and 39 AVFs (75.0%) were prepared for immediate puncturing. Catheter insertion was required in 12 cases (23.1%). These cases were comprised of 1 failed case and of 11 cases that was not adequate for immediate cannulation (PTFE graft interposition, basilic vein elevation). Average procedure times were 121.8 ┬▒ 63.5 minutes. Except 1 case of post-operative minor bleeding, no other complications were developed. Hospital admission required in 11 patients (median hospital stays, 0 [0ŌĆō2] days) but in most patients (41 patients), the procedures were done at out-patient setting (Table 2). A total of 19 procedures (2 surgery, 17 endovascular procedures) for 6 (11.5%) patients were performed to maintain secondary patency in AVF over median follow up 21.5 (0.0ŌĆō114.9) months. Multiple procedures were done to remain access patency in small number of patients (such as 5 to 8 angioplasties in one patient). First intervention months (Table 3) after surgical reconstruction was 5.6 (1.3ŌĆō82.8) months (median). The re-intervention rate was 0.27 ┬▒ 0.92/AVF patient/year (mean ┬▒ standard deviation), or 0 (0ŌĆō5, median). The primary and secondary patency rates for AVF at 6, 12, 18, and 24 months were 88.5%, 83.2%, 83.2% and 83.2 % and 96.0%, 96.0%, 93.2%, and 93.2% (Fig. 1), respectively.
There are a few guidelines about managing AVF thrombosis. The National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines [8] recommend that, for cases of thrombosed AVF, surgery is recommended only when the stenosis is in the juxta-anastomotic area and recurrent natures. According to the European Best Practice Guidelines (EBPG) [9], thrombosed AVFs should be treated with either interventional radiology or surgery. They further recommend that each treatment center should review their results and select the modality that has produced the best outcomes. Mickley [5] recommended surgical treatment for most AVF thromboses because thrombosis rarely occurs as a type-2 stenosis. Most thromboses occur as juxta-anastomotic or junctional area stenosis. In this study, most cases were juxta-anastomotic stenosis. At this center, outflow vein stenosis was most commonly treated percutaneously. Our cases that received surgery for outflow vein stenosis had previously undergone multiple angioplasties or were co-occurring with an aneurysm; therefore, surgical reconstruction was considered for these cases. Most thrombosis cases caused by cephalic-arch stenosis are now treated by endovascular thrombectomy and balloon dilation [10]. In a few cases where the endovascular approach failed or where the stenosis involved a diffuse obliterated vein, the chosen treatment method involved rerouting to the brachial or basilica vein. In this study, all 3 cases had previous angioplasties (successful or failed). In two of the cases, no recurrent stenosis developed, but in one case, repeat stenosis developed and required multiple angioplasties without access to the thrombosis. Especially in developing, low-income countries, surgical reconstruction has many advantages compared to percutaneous treatment if intervention results are comparable. Above all, endovascular intervention is more costly than surgery. In South Korea, fistula creation or revision insurance fees are about US dollar (USD) 480.24 compared to balloon angioplasty, which can cost about USD 809.94 [11]. In addition to insurance costs, high equipment costs, including the balloon, sheath, guidewire, and the fluoroscopic machine, must also be considered. Consequently, surgical thrombectomies are more prevalent in low-income countries [12].
The overall technical success rate was 98.1%, which is higher than that of previously published data for open thrombectomies for autologous and prosthetic accesses [13ŌĆō19]. The catheter placement rate was 23%, which is similar to the average rate (26%) reported in a meta-analysis of prospective studies [16]. In the report of Chen et al [6], the cumulative patency rates for 27 surgical procedures were 70% and 57% at 6 and 12 months, respectively. In other reports [2], the cumulative patency rates for 167 surgical procedures were 84% and 70% at 6 and 12 months, respectively. The results (primary and secondary patency rates for AVF at 6, 12, 18, and 24 months were 88.5%, 83.2%, 83.2% and 83.2% and 96.0%, 96.0%, 93.2%, and 93.2%, respectively) were similar or better than those of these previous reports [2,6]. But, selection bias was factor to be considered because this study was retrospective nature.
The salvage of thrombosed AVFs and grafts by an interventional radiologist had an initial success rate of 78ŌĆō98%, while primary and secondary patency rates at 12 months were 34ŌĆō50% and 80ŌĆō86%, respectively [20]. In addition to the above studies, most studies [21] that have compared results from thrombectomy treatments (surgical vs. endovascular) concluded that primary patency was high in surgical intervention, and that re-intervention procedures were common after endovascular treatment, but that secondary patencies showed no difference after effective multiple angioplasties. The collected data suggest that the benefit of surgical thrombectomy was seen mainly when the surgical method combined thrombectomy with some form of revision/interposition. In this study, the author only evaluated autogenous fistulas, and most cases underwent reanastomosis procedures. These were the factors that were associated with high success rates and high long-term survival in this study. Only two cases received a thrombectomy with angioplasty but without reconstruction. Among them, one case experienced operation failure. Surgical thrombectomy for autogenous AVFs is often thought to be a challenging and fruitless endeavor. But adequate information of patent vein and stenosis detection leads to high operation success rates. Among the few failure cases in this study, inadequate preoperative mapping or unsuccessful management of underlying stenotic lesions was the cause of failure. This was minimized by preoperative or intraoperative ultrasonography (USG) or angiographic stenosis detection. Using only precise preoperative mapping, the author was able to perform simple reanastomosis in the proximal or upper-elbow area with excellent success rates. Excellent primary results after angioplasty for stenosed AVF have been shown to be associated with a high recurrence rate [20]. Therefore, easily accessible lesions should be treated surgically. The decision to take an endovascular approach versus surgical revision should be determined based on the underlying lesion (stenosis or no stenosis), length of the thrombosed fistula, patencies of distal runoff veins, availability of the inflow artery, and physicianŌĆÖs experience level. Most cases of surgical revision require patent distal runoff veins, stenotic lesions that are limited to a focal segment, or a fistula thrombosis that is limited to a short segment. Therefore, the decision to take an endovascular approach or to perform surgical revision requires a thorough physical examination and precise ultrasound imaging. At this center, a dedicated interventional nephrologist routinely performed vascular access ultrasound for preoperative mapping, stenosis detection, and brachial artery flow monitoring. This is one reason why it is necessary for the nephrologist to be involved in access revision. Timely salvage of the failed vascular access is very important to restore function of the AVF and continue its use and to reduce the need for a hemodialysis catheter. Also, prolonged thromboses can become organized, and venous intimal changes can lead to recanalization failure. Most cases dialyzed immediately by newly reconstructed access without requiring catheter insertion in this study. Surgical salvage of AVFs was usually performed within one dialysis day after thrombosis detection. No surgical or radiologic consultations were performed; thus, time delays were minimized. The average elapsed time between thrombosis and surgery was 1.7 days.
Interventional nephrology has emerged as a subspecialty worldwide, but surgical activity is still limited to a few individual areas [12,22,23]. These studies, however, have reported high success rates of access creation and management. Herein, the author found that simple surgical procedures can be performed successfully and safely by an interventional nephrologist. Local anesthesia was successfully used in all thrombectomies and reanastomoses, rather than general anesthesia, eliminating the need for preoperative fasting (convenient for diabetics), an anesthesia team, or operating-theater equipment. Why simple?; because this surgical procedures rarely require fluoroscopic machine, only 1 case required intra-operative USG and only required local anesthesia, small amount of incision. In several Korean reports [3,24,25], more complex surgeries such as fluoroscopic monitoring, use of expensive balloon were required despite of preservation of venous reserve. The author was also able to use the operating room or preparatory room at any time of the day. After surgery, patients were usually discharged from the emergency room after some hours, and hospitalization was not required.
However, the case sample size was too small and the observation period was too short for relevant conclusions to be drawn from these otherwise promising results.
The author concluded from this experience that surgical salvage of thrombosed AVFs, when performed under local anesthesia by a skilled interventional nephrologist and after conducting adequate preoperative USG, can be successful in the short- and long-term, and the author strongly recommends it.
Figure┬Ā1
Kaplan-Meier analysis shows primary patency and secondary patency after surgical salvage procedures.
Table┬Ā1
Baseline characteristics
Table┬Ā2
Results of surgical management of thrombosed forearm and upper arm fistulas
References
1. Cohen A, Korzets A, Neyman H, Ori Y, Baytner S, Belenky A, Knieznik M, Bachar GN, Atar E. Endovascular interventions of juxtaanastomotic stenoses and thromboses of hemodi-alysis arteriovenous fistulas. J Vasc Interv Radiol 20:66ŌĆō70. 2009;


2. Ponikvar R. Surgical salvage of thrombosed arteriovenous fistulas and grafts. Ther Apher Dial 9:245ŌĆō249. 2005;


3. Hyun JH, Lee JH, Park SI. Hybrid surgery versus percutaneous mechanical thrombectomy for the thrombosed hemodialysis autogenous arteriovenous fistulas. J Korean Surg Soc 81:43ŌĆō49. 2011;



4. Tessitore N, Mansueto G, Lipari G, Bedogna V, Tardivo S, Baggio E, Cenzi D, Carbognin G, Poli A, Lupo A. Endovascular versus surgical preemptive repair of forearm arteriovenous fistula juxta-anastomotic stenosis: analysis of data collected prospectively from 1999 to 2004. Clin J Am Soc Nephrol 1:448ŌĆō454. 2006;


5. Mickley V. Stenosis and thrombosis in haemodialysis fistulae and grafts: the surgeonŌĆÖs point of view. Nephrol Dial Transplant 19:309ŌĆō311. 2004;


6. Chen JC, Kamal DM, Jastrzebski J, Taylor DC. Venovenostomy for outflow venous obstruction in patients with upper extremity autogenous hemodialysis arteriovenous access. Ann Vasc Surg 19:629ŌĆō635. 2005;


7. Oakes DD, Sherck JP, Cobb LF. Surgical salvage of failed radiocephalic arteriovenous fistulae: techniques and results in 29 patients. Kidney Int 53:480ŌĆō487. 1998;


8. National Kidney Foundation K/DOQI. Clinical practice guidelines for vascular access. 2006 updates Available at: http://www2.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA. Data accessed: 10 August 2016.

9. Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R. EBPG on vascular access. Nephrol Dial Transplant 22(Suppl 2):ii88ŌĆōii117. 2007;


10. Vasanthamohan L, Gopee-Ramanan P, Athreya S. The management of cephalic arch stenosis in arteriovenous fistulas for hemodialysis: a systematic review. Cardiovasc Intervent Radiol 38:1179ŌĆō1185. 2015;


11. Health Insurance Review and Assessment Service. Health insurance benefit cost 2016 Available at: http://www.hira.or.kr/ebook/38cca21b-f749-47cd-a906-e0321b078d46/595.html. Data accessed: 10 August 2016.
12. Sampathkumar K, Lobo V, Balasubramaniam J, Mahaldar A, Yevzlin AS, Kumbar L. Vascular access creation and care--Perspective from India. Adv Chronic Kidney Dis 22:466ŌĆō470. 2015;


13. Cull DL, Washer JD, Carsten CG, Keahey G, Johnson B. Description and outcomes of a simple surgical technique to treat thrombosed autogenous accesses. J Vasc Surg 56:861ŌĆō865. 2012;


14. Tordoir JH, Bode AS, Peppelenbosch N, van der Sande FM, de Haan MW. Surgical or endovascular repair of thrombosed dialysis vascular access: is there any evidence? J Vasc Surg 50:953ŌĆō956. 2009;


15. Lipari G, Tessitore N, Poli A, Bedogna V, Impedovo A, Lupo A, Baggio E. Outcomes of surgical revision of stenosed and thrombosed forearm arteriovenous fistulae for haemodialysis. Nephrol Dial Transplant 22:2605ŌĆō2612. 2007;


16. Kuhan G, Antoniou GA, Nikam M, Mitra S, Farquharson F, Brittenden J, Chalmers N. A meta-analysis of randomized trials comparing surgery versus endovascular therapy for thrombosed arteriovenous fistulas and grafts in hemodialysis. Cardiovasc Intervent Radiol 36:699ŌĆō705. 2013;


17. Ponikvar R. Surgical salvage of thrombosed native arteriovenous fistulas for hemodialysis by interventional nephrologists. Ther Apher Dial 13:340ŌĆō344. 2009;


18. Palmer RM, Cull DL, Kalbaugh C, Carsten CG, Taylor SM, Snyder BA, York JW, Langan EM, Blackhurst D. Is surgical thrombectomy to salvage failed autogenous arteriovenous fistulae worthwhile? Am Surg 72:1231ŌĆō1233. 2006.


19. Marston WA, Criado E, Jaques PF, Mauro MA, Burnham SJ, Keagy BA. Prospective randomized comparison of surgical versus endovascular management of thrombosed dialysis access grafts. J Vasc Surg 26:373ŌĆō380. 1997;


20. Turmel-Rodrigues L, Pengloan J, Baudin S, Testou D, Abaza M, Dahdah G, Mouton A, Blanchard D. Treatment of stenosis and thrombosis in haemodialysis fistulas and grafts by interventional radiology. Nephrol Dial Transplant 15:2029ŌĆō2036. 2000;


21. Napoli M, Prudenzano R, Russo F, Antonaci AL, Aprile M, Buongiorno E. Juxta-anastomotic stenosis of native arteriovenous fistulas: surgical treatment versus percutaneous transluminal angioplasty. J Vasc Access 11:346ŌĆō351. 2010;


22. Mishler R, Yang Z, Mishler E. Arteriovenous fistula creation by nephrologist access surgeons worldwide. Adv Chronic Kidney Dis 22:425ŌĆō430. 2015;


23. Mishler R. Autologous arteriovenous fistula creation by nephrologists. Adv Chronic Kidney Dis 16:321ŌĆō328. 2009;





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















