| Kidney Res Clin Pract > Volume 36(1); 2017 > Article |
|
Abstract
Background
We investigated the long-term effect of AST-120, which has been proposed as a therapeutic option against renal disease progression, in patients with advanced chronic kidney disease (CKD).
Methods
We performed post-hoc analysis with a per-protocol group of the K-STAR study (Kremezin study against renal disease progression in Korea) that randomized participants into an AST-120 and a control arm. Patients in the AST-120 arm were given 6 g of AST-120 in three divided doses, and those in both arms received standard conventional treatment.
Results
The two arms did not differ significantly in the occurrence of composite primary outcomes (log-rank P = 0.41). For AST-120 patients with higher compliance, there were fewer composite primary outcomes: intermediate tertile hazard ratio (HR) 0.62, 95% confidence interval (CI) 0.38 to 1.01, P = 0.05; highest tertile HR 0.436, 95% CI 0.25 to 0.76, P = 0.003. The estimated glomerular filtration rate level was more stable in the AST-120 arm, especially in diabetic patients. At one year, the AST-120-induced decrease in the serum indoxyl sulfate concentration inversely correlated with the occurrence of composite primary outcomes: second tertile HR 1.59, 95% CI 0.82 to 3.07, P = 0.17; third tertile HR 2.11, 95% CI 1.07 to 4.17, P = 0.031. Furthermore, AST-120 showed a protective effect against the major cardiovascular adverse events (HR 0.51, 95% CI 0.26 to 0.99, P = 0.046).
The chronic kidney disease (CKD) prevalence in Korean adults was 7.2% in 2007 according to the Korean National Health and Nutrition Examination Survey [1]. The incidence of end stage renal disease (ESRD) is increasing at a rate of 10% or more per year in Korea [2], although angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) effectively preserve the serum creatinine (SCr) and prevent progression to ESRD or death [3–5]. Thus, we need a new option to improve renal outcomes and to lessen the socioeconomic burden of CKD.
Dietary protein-derived tryptophan is metabolized into indole, which is then absorbed into the blood stream and oxidized into indoxyl sulfate (IS) in the liver [6]. CKD patients have elevated serum IS levels because of reduced renal excretion of IS [7,8], and this disease type causes elevated expression of genes related to tubulointerstitial fibrosis, such as those coding for transforming growth factor β1 and collagen [9,10]. AST-120 (Kremezin®, Kureha Corporation, Tokyo, Japan) adsorbs uremic toxins and precursors, including indole, and excretes them into feces.
AST-120 reduced glomerular sclerosis and the SCr levels [11–13]. Additionally it has improved uremic symptoms and renal functional deterioration [14–16]. In the Effect of a carboneous oral adsorbent on the progression of CKD (CAP-KD) trial, researchers found no significant difference in the primary composite outcomes, but the estimated glomerular filtration rate (eGFR) decreased less in the AST-120 arm [17]. However, the study was small (n = 460) and only covered one year. Evaluating Prevention on Progression in Chronic Kidney Disease (EPPIC-1 and 2) trials also showed the same trend [18]. In addition, the K-STAR study (Kremezin study against renal disease progression in Korea) showed negative results with respect to the composite primary outcome [19].
Here, we re-analyzed the K-STAR study with a per-protocol group to clarify the long-term effect of AST-120 on renal disease progression and to characterize patients who benefit from AST-120.
K-STAR was a prospective, 11-center, randomized, open-label, controlled study. Participants recruited from March 2009 to August 2010 were followed up for 36 months (clinicaltrials.gov: NCT00860431). The primary outcome was a composite of SCr doubling, 50% reduction of eGFR, or initiation of renal replacement therapy. Secondary outcomes were (1) the rate of eGFR changes (Δ eGFR/month), (2) changes in the urinary protein excretion, (3) all-cause mortality, (4) all-cause hospitalization other than planned surgery or interventions, and (5) changes in the health-related quality of life. We prospectively collected data about severe adverse events including diagnosis and progress as well as event period. We compared the major adverse cardiovascular events (MACE) (myocardial infarction, unstable angina, cardiovascular death, revascularization, fatal/non-fatal accident, peripheral arteriopathy, and aortic events) between the two treatment arms.
The eligibility criteria, randomization, interventions, and measurements from the K-STAR study were introduced in a previously published article [19]. We performed the post-hoc analysis in the per-protocol participants, who were defined as those who completed the trial without major protocol violations.
This study was an investigator-initiated one using data from the K-STAR study, and participants allowed us to perform further analyses using their data when they provided the informed consent. The protocol was approved by the institutional review board of Seoul National University Hospital (IRB approval number 1606-073-711). We conducted this study in compliance with the principles of the Declaration of Helsinki.
We performed the post-hoc analysis in the per-protocol participants, defined as those who completed the trial without major protocol violations. We used SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). We used Student’s t-test and Wilcoxon signed rank test to determine the means and standard deviations for continuous variables. The chi-square test was used for categorical variables, Kaplan-Meier survival analysis to determine the cumulative survival probability, and the log-rank test to test the survival difference between the two arms. Multivariate Cox proportional-hazard regression analysis based on the enter method was also applied. We used a mixed model to test the within- and between-individual differences of repeatedly measured eGFR change rates between the two arms. We considered P < 0.05 (two-sided) as statistically significant.
We evaluated 465 patients in the per-protocol group (Fig. 1).
The mean age of the analyzed patients was 57 years, and 67.5% were men. Diabetic nephropathy was reported in 229 (49.2%) patients. Systolic and diastolic blood pressure values were 129 ± 15.3/76 ± 9.9 mmHg. The mean SCr level was 247.5 ± 59.23 μmol/L and mean eGFR was 26.8 ± 7.26 mL/min/1.73m2. The mean urinary protein excretion rate was 2.0 ± 2.01 g/g Cr. ACE inhibitors or ARBs were taken by 416 (89.5%), beta-blockers by 245 (52.7%), calcium channel blockers by 316 (68.0%), diuretics by 284 (61.1%), and lipid modifiers by 321 (69.0%). The baseline characteristics did not differ significantly between two arms (Table 1).
By the end of the study period, 194 patients (41.7% of analyzed participants) reached a primary outcome. A SCr doubling or a > 50% eGFR reduction was observed in 120 patients (57 control and 63 AST-120 cases), and renal replacement therapy was initiated in 74 patients (40 control, 34 AST-120). The two arms did not differ significantly in their cumulative rate of composite primary outcome events (log-rank P = 0.41) (Fig. 2). Cox proportional-hazard analysis showed that diabetic nephropathy and the severity of kidney dysfunction are risk factors for the occurrence of a primary outcome (Supplementary table 1).
When we divided patients according to the AST-120 medication compliance, the highest tertile was more than 96.5% and lowest tertile was less than 90.9%. Baseline characteristics based on the AST-120 compliance were not statistically different (Table 2). Higher AST-120 compliance lessened the occurrence of composite primary outcomes when we compared three tertiles of AST-120 compliance (breslow P = 0.038, log-rank P = 0.019), even after adjusting for diabetic nephropathy, proteinuria, and eGFR: intermediate tertile hazard ratio (HR) 0.62, 95% confidence interval (CI) 0.38 to 1.01, P = 0.05; highest ter-tile HR 0.44, 95% CI 0.25 to 0.76, P = 0.003 (Table 3).
Analyzed in conjunction with time (Prandomization = 0.18, Prandomization-time = 0.04), the loss of renal function was delayed in the AST-120 arm (Fig. 3A). The beneficial effect of AST-120 was significant in the absence of composite primary outcomes (Prandomization = 0.01, Fig. 3B), but not in their presence (Prandomization = 0.28, Fig. 3C). AST-120 was effective in patients with diabetic nephropathy when we analyzed the patients in conjunction with time (Prandomization = 0.54, Prandomization-time = 0.049, Fig. 3D). However, AST-120 did not show significant efficacy in patients with non-diabetic nephropathy (Pran-domization = 0.21, Fig. 3E). The slope of 1/SCr significantly decreased in both arms after randomization during the study period compared with the screening period. The value of the AST-120 arm (−0.0008 ± 0.0028) was much less than that of the control arm (−0.0015 ± 0.0028) (P = 0.046) (Supplementary fig. 1).
The urinary protein excretion rate was also decreased during the study period (from 2.0 ± 1.98 g/g Cr to 1.8 ± 2.02 g/g Cr in the control arm, and from 2.0 ± 2.05 g/g Cr to 1.2 ± 1.20 g/g Cr in the AST-120 arm). The amount of daily proteinuria at the last visit was significantly lower in the AST-120 arm (P = 0.007). The two arms did not significantly differ in all-cause mortality, hospitalizations other than planned surgery or intervention, or health-related quality of life.
The serum and urine IS levels were significantly lower in the AST-120 arm than in the control arm throughout the study period. We calculated the ratio of the serum IS level one year after AST-120 treatment and compared it to the level at the time of randomization. The first tertile of the ratio was < 0.6825 and the third was > 2.0000. Daily proteinuria one year after randomization was lower in the first tertile (1.6 ± 1.94 vs. 2.3 ± 2.74 g/g Cr, P = 0.038). Additionally, the cumulative rate of the composite primary outcomes was significantly lower in the first tertile than in the others, in sequence (log-rank P = 0.033) (Fig. 4). Less change in the serum IS level, as well as lower eGFR and more urinary protein excretion were risk factors for the occurrence of a composite primary outcome: second tertile HR 1.59, 95% CI 0.82 to 3.07, P = 0.17; third tertile HR 2.11, 95% CI 1.07 to 4.17, P = 0.031 (Table 4).
A total of 40 MACE cases occurred during the study period (13 in the AST-120 arm vs. 27 in the control arm; P = 0.046). The cumulative rate of MACE was less in the AST-120 arm than in the control arm (Breslow P = 0.031, log-rank P = 0.045). Cox proportional-hazards analysis showed the protective effect of AST-120 on the occurrence of MACE after adjusting for diabetic nephropathy, proteinuria, and CKD stage (HR 0.51, 95% CI 0.26 to 0.99, P = 0.046) (Table 5).
While the AST-120 combination proved to be more protective of renal function (eGFR and slope of 1/SCr) than standard treatment alone, especially in patients with diabetic nephropathy, AST-120 did not delay the onset of composite primary outcomes. However, greater reduction in the serum IS, as well as high AST-120 compliance in the AST-120 arm, were related to the lower composite primary outcome occurrence. AST-120 also had a protective effect on the occurrence of MACEs.
IS is known to be associated with renal disease progression through increased expression of fibrogenic genes and oxidative stress [12,20–22] and with klotho down-regulation [23]. AST-120 was effective in lowering the serum and urinary levels of IS and advanced glycation end products (AGEs) in pre-clinical and clinical studies [21,22,24,25]. In addition, AST-120 might ameliorate uremic complications by increasing the renal expression of klotho in CKD patients [26].
In the present study, AST-120 did not specifically demonstrate that AST-120 has a beneficial effect on composite primary outcomes in CKD patients. In participants whose renal disease was too far advanced (mean eGFR, 26 mL/min/1.73m2) for AST-120 to reverse its progression and the intervention period was relatively short (≤ 36 months). In a previous study, the greatest preservation of renal function was observed in patients who had been receiving AST-120 the longest (> 30 months) [27]. In fact, the median duration of AST-120 administration in both arms was 19 months for those who reached the composite primary outcome and 33 months for those who did not
AST-120 preserved eGFR in diabetic nephropathy patients. In CKD patients, AST-120 treatment decreases the serum and urinary levels of AGEs [24,25]. The formation of AGEs is increased when there is hyperglycemia and oxidative stress, such as in uremic conditions. AGEs induce cellular responses that include up-regulation of profibrogenic and proinflammatory cytokines, leading to progressive nephropathies. Renal dysfunction increases the level of circulating AGEs due to both the reduced clearance and increased formation of AGEs. Because those effects are prominent in diabetic nephropathy [24,25,28], they may explain the favorable effect of AST-120 in patients with that condition.
Our finding that a higher reduction ratio of the serum IS improves the clinical outcome (reduced daily protein-uria and fewer primary outcomes) was significant, even after adjusting for renal function and urinary protein excretion. Additionally, daily proteinuria one year after randomization was lower in the higher serum IS reduction group. Residual albuminuria during therapy was reported as a strong marker of poor renal outcome, and reducing proteinuria as much as possible is usually recommended [29]. Alleviation of inflammation and oxidative stress via IS reduction reduces the urinary protein excretion and improves the prognosis. AST-120 compliance among three tertiles of the serum IS ratio during 1 year did not differ (1st tertile, 93%; 2nd tertile, 92%; and 3rd tertile, 91%) (P = 0.445). Patients’ condition related to the serum IS response to AST-120 including inflammation and oxidative stress may have importance, as well as AST-120 compliance itself.
This study also shows that AST-120 has favorable effects on the MACE in advanced CKD patients, although we could not determine the patients’ cardiovascular events prior to study participation. Previous reports declared that AST-120 ameliorated aortic calcification, left ventricular mass, and cardiac fibrosis [30–32]. These findings indicate the clinical importance of uremic toxin control given the large burden of cardiovascular morbidity and mortality associated with CKD patients. Future studies about the relationship between serum IS reduction and cardiovascular outcomes in patients taking AST-120 may be of considerable interest.
This study has a limitation in the study design itself. We cannot extrapolate the results to entire advanced CKD patients because this study selected a per-protocol group of patients. Patients who did not complete the study may have their own reasons for not adhering to the protocol or may have developed complications related to the AST-120 treatment. As a strength, our study suggests a novel approach for selecting patient subgroups likely to benefit from AST-120 treatment. AST-120 slowed renal function deterioration, especially benefiting patients with diabetic nephropa-thy and higher AST-120 compliance was related with better renal outcome. Regular measurement of the serum IS during follow-up is valuable and should be implemented to evaluate treatment response and compliance.
In conclusion, long-term use of AST-120 delays renal function deterioration in advanced CKD patients. Furthermore the beneficial effect is more evident in patients with diabetic nephropathy. AST-120 also had a protective effect against the occurrence of cardiovascular events. The serum IS concentration may have clinical significance as a marker for predicting renal disease progression. A longer clinical trial with patients at an earlier stage of kidney disease should be instituted to clarify the clinical usefulness of AST-120.
Figure 1
Diagram of participant enrollment and analysis, the Kremezin study against renal disease progression in Korea (K-STAR).
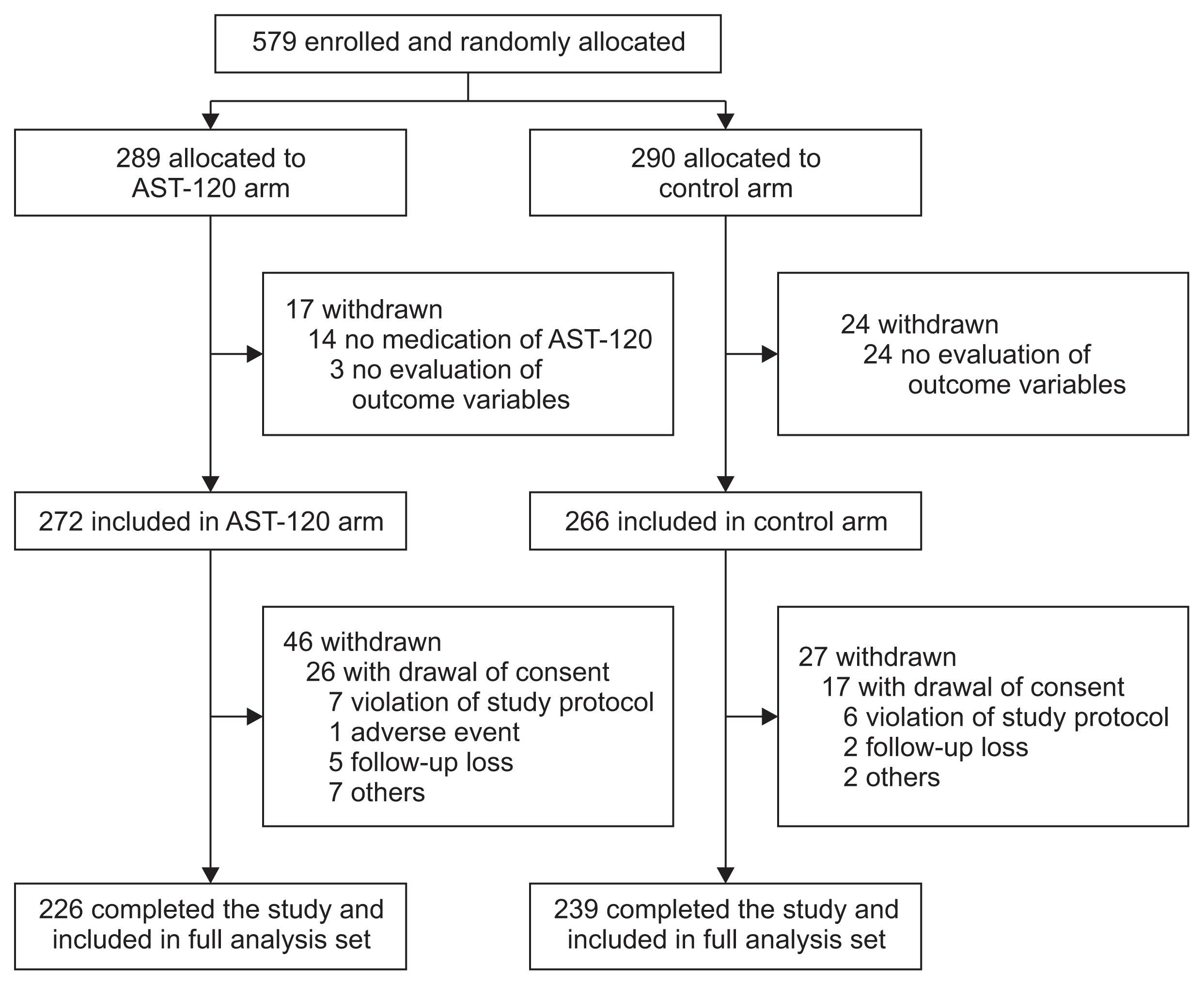
Figure 3
Change of estimated glomerular filtration rate (eGFR) over time
(A) From whole per-protocol participants (Prandomization = 0.18, Prandomization-time = 0.04). (B) From participants without a composite primary outcome (Prandomization = 0.01). (C) From participants with a composite primary outcome (Prandomization = 0.28). (D) From participants with diabetic nephropathy (Prandomization = 0.54, Prandomization-time = 0.049). (E) From participants with non-diabetic nephropathy (Prandomization = 0.21).
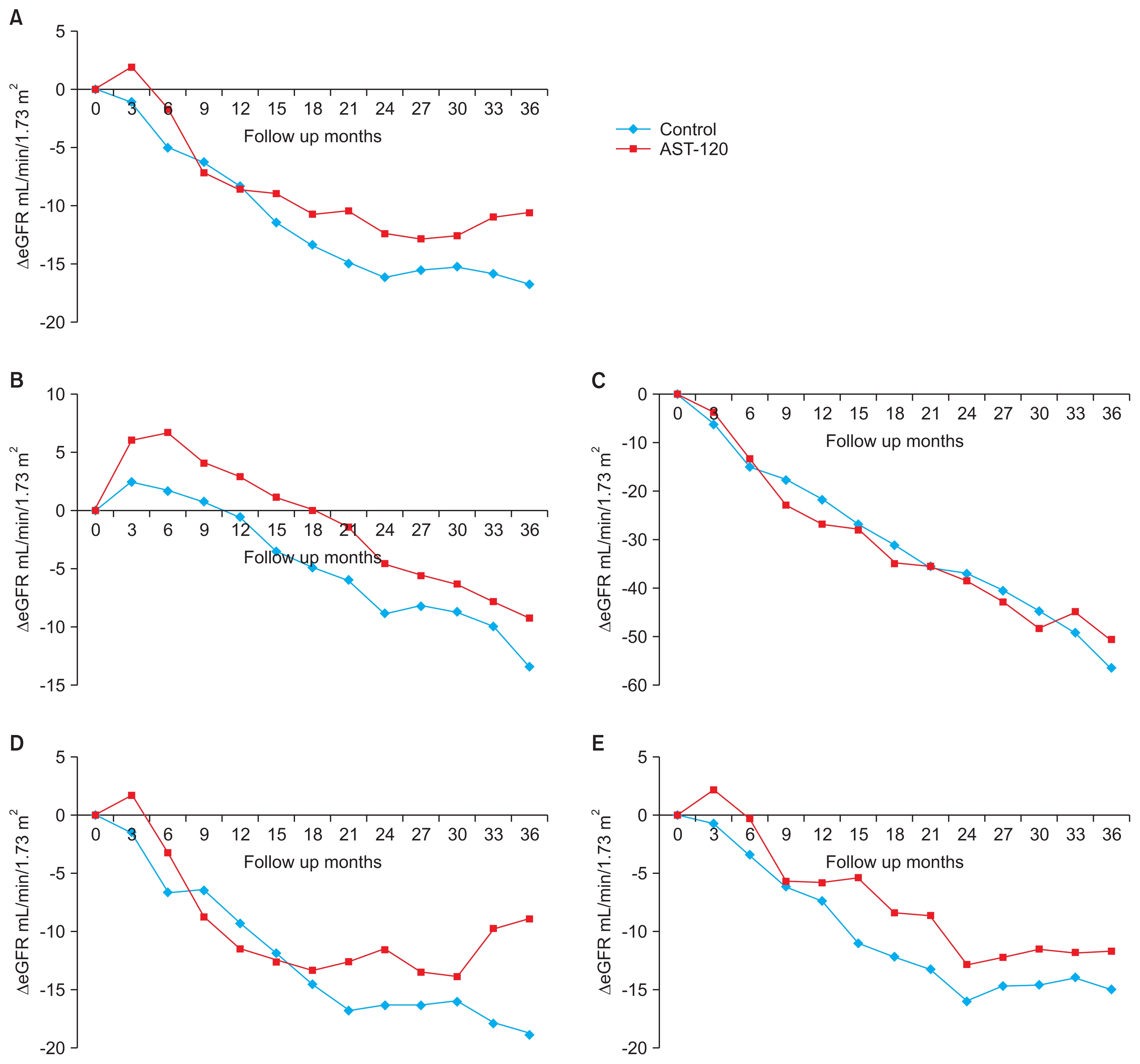
Figure 4
The effect of the change of serum indoxyl sulfate level on primary outcome in the AST-120 arm
Log-rank P = 0.033. First tertile, serum indoxyl sulfate (one year/at the time of randomization) < 0.6825; third tertile, serum indoxyl sulfate (one year/at the time of randomization) > 2.0000.

Table 1
Baseline characteristics of per-protocol participants
| Variable | Control (n = 239) | AST-120 (n = 226) | P |
|---|---|---|---|
| Age (yr) | 57 ± 12.9 | 56 ± 13.4 | 0.51 |
| Gender (female:male) | 81/158 (33.9/66.1) | 70/156 (31.0/69.0) | 0.55 |
| ESRD cause | 0.85 | ||
| Diabetic | 119 (49.8) | 110 (48.7) | |
| Non-diabetic | 120 (50.2) | 116 (51.3) | |
| BMI (kg/m2) | 24.5 ± 3.38 | 24.8 ± 3.84 | 0.37 |
| BSA (m2) | 1.7 ± 0.18 | 1.7 ± 0.17 | 0.59 |
| SBP (mmHg) | 129.5 ± 15.97 | 129.5 ± 14.59 | 0.97 |
| DBP (mmHg) | 75.6 ± 9.94 | 76.1 ± 9.78 | 0.57 |
| Serum Cr (μmol/L) | 248.4 ± 60.91 | 247.5 ± 56.66 | 0.89 |
| eGFR (mL/min/1.73m2) | 26.6 ± 7.07 | 27.1 ± 7.47 | 0.46 |
| CKD stage 3/4 | 78/161 (32.6/67.4) | 72/154 (31.9/68.1) | 0.86 |
| Urinary protein (g/g Cr) | 2.0 ± 1.98 | 1.97 ± 2.05 | 0.73 |
| Hemoglobin (g/L) | 113.0 ± 17.20 | 115.0 ± 19.70 | 0.28 |
| Albumin (g/L) | 39.9 ± 4.91 | 40.4 ± 4.00 | 0.30 |
| Uric acid (μmol/L) | 480.0 ± 97.25 | 494.3 ± 123.30 | 0.17 |
| LDL (mmol/L) | 2.4 ± 0.78 | 2.4 ± 0.72 | 0.51 |
| CRP (mg/dL) | 0.9 ± 3.32 | 0.7 ± 2.51 | 0.49 |
| Serum β2–MG (mg/L) | 6.8 ± 2.50 | 6.5 ± 2.26 | 0.21 |
| Serum IS (mg/dL) | 0.7 ± 0.84 | 0.6 ± 0.55 | 0.19 |
| Urine IS (mg/dL) | 7.6 ± 7.66 | 6.7 ± 5.17 | 0.11 |
| RAS inhibitor | 211 (88.3) | 205 (90.7) | 0.45 |
| Beta-blocker | 121 (50.6) | 124 (54.9) | 0.40 |
| Ca++ channel blocker | 163 (68.2) | 153 (67.7) | 0.92 |
| Diuretics | 138 (57.7) | 146 (64.6) | 0.16 |
| Lipid modifier | 160 (67.0) | 161 (71.2) | 0.37 |
β2–MG, beta2–microglobulin; BMI, body mass index; BSA, body surface area; CKD, chronic kidney disease; Cr, creatinine; CRP, C–reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; IS, indoxyl sulfate; LDL, low-density lipoprotein; RAS, rennin-angiotensin-aldosterone system; SBP, systolic blood pressure.
Table 2
Baseline characteristics based on the AST-120 medication compliance
| Variable | Lowest tertile (n = 75) | Intermediate (n = 76) | Highest tertile (n = 74) | P |
|---|---|---|---|---|
| Age (yr) | 56 ± 12.9 | 55 ± 13.9 | 59 ± 12.6 | 0.06 |
| Gender (female:male) | 23/52 (30.7/69.3) | 20/56 (26.3/73.7) | 27/47 (36.5/63.5) | 0.41 |
| ESRD cause | 0.76 | |||
| Diabetic | 34 (45.3) | 39 (51.3) | 37 (50.0) | |
| Non-diabetic | 41 (54.7) | 37 (48.7) | 37 (50.0) | |
| BMI (kg/m2) | 24.2 ± 3.73 | 25.3 ± 3.88 | 24.96 ± 3.91 | 0.22 |
| BSA (m2) | 1.7 ± 0.17 | 1.8 ± 0.17 | 1.7 ± 0.18 | 0.24 |
| SBP (mmHg) | 127.2 ± 14.53 | 130.2 ± 16.00 | 131.4 ± 13.00 | 0.20 |
| DBP (mmHg) | 75.4 ± 8.96 | 76.5 ± 10.61 | 76.6 ± 9.84 | 0.74 |
| Serum Cr (μmol/L) | 251.9 ± 58.79 | 255.5 ± 60.996 | 235.1 ± 47.82 | 0.08 |
| eGFR (mL/min/1.73m2) | 26.6 ± 7.55 | 27.2 ± 6.68 | 27.2 ± 8.23 | 0.84 |
| CKD stage 3/4 | 23/52 (30.7/69.3) | 26/50 (34.2/65.8) | 22/52 (29.7/70.3) | 0.83 |
| Urinary protein (g/g Cr) | 1.9 ± 1.87 | 1.9 ± 1.97 | 2.2 ± 2.32 | 0.54 |
| Hemoglobin (g/L) | 112.0 ± 20.50 | 115.0 ± 16.80 | 116.0 ± 21.50 | 0.38 |
| Albumin (g/L) | 40.3 ± 3.95 | 40.5 ± 3.75 | 40.4 ± 4.39 | 0.97 |
| Uric acid (μmol/L) | 507.96 ± 129.96 | 490.7 ± 114.68 | 481.2 ± 122.77 | 0.19 |
| LDL (mmol/L) | 2.3 ± 0.72 | 2.3 ± 0.76 | 2.5 ± 0.69 | 0.19 |
| CRP (mg/dL) | 0.8 ± 1.78 | 1.0 ± 3.82 | 0.4 ± 0.80 | 0.28 |
| Serum β2–MG (mg/L) | 6.6 ± 2.16 | 6.6 ± 2.28 | 6.4 ± 2.28 | 0.75 |
| Serum IS (mg/dL) | 0.6 ± 0.53 | 0.7 ± 0.58 | 0.6 ± 0.51 | 0.79 |
| Urine IS (mg/dL) | 6.4 ± 4.66 | 7.2 ± 5.79 | 6.54 ± 5.01 | 0.63 |
β2–MG, beta2–microglobulin; BMI, body mass index; BSA, body surface area; CKD, chronic kidney disease; Cr, creatinine; CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; IS, indoxyl sulfate; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Table 3
Cox proportional-hazard analysis for composite primary outcome according to AST-120 compliance in the AST-120 arm
| Variable | Composite primary outcome | ||
|---|---|---|---|
|
|
|||
| HR | 95% CI | P | |
| Age (yr) | 0.95 | 0.93–0.97 | < 0.001 |
| Gender (female)* | 0.71 | 0.45–1.12 | 0.14 |
| CKD cause (diabetic nephropathy)† | 1.10 | 0.64–1.89 | 0.72 |
| Urinary protein (g/g Cr) | 1.68 | 1.50–1.88 | < 0.001 |
| eGFR | 0.89 | 0.86–0.93 | < 0.001 |
| Intermediate tertile of AST-120 compliance | 0.62 | 0.38–1.01 | 0.05 |
| Highest tertile of AST-120 compliance‡ | 0.44 | 0.25–0.76 | 0.003 |
Table 4
Cox proportional-hazard analysis for composite primary outcome according to change in serum indoxyl sulfate level over 1 year in the AST-120 arm
| Variable | Composite primary outcome | ||
|---|---|---|---|
|
|
|||
| HR | 95% CI | P | |
| Age (yr) | 0.94 | 0.92–0.96 | < 0.001 |
| Gender (female)* | 0.66 | 0.38–1.14 | 0.14 |
| CKD cause (diabetic nephropathy)† | 0.94 | 0.48–1.85 | 0.86 |
| Urinary protein (g/g Cr) (12 mo) | 1.32 | 1.20–1.45 | < 0.001 |
| eGFR (12 mo) | 0.79 | 0.75–0.84 | < 0.001 |
| Second tertile of serum IS ratio | 1.59 | 0.82–3.07 | 0.17 |
| Third tertile of serum IS ratio‡ | 2.11 | 1.07–4.17 | 0.031 |
Table 5
Cox proportional-hazard analysis for major adverse cardiovascular events
| Variable | Major adverse cardiovascular events | ||
|---|---|---|---|
|
|
|||
| HR | 95% CI | P | |
| Age (yr) | 1.05 | 1.02–1.08 | 0.002 |
| Gender (female)* | 0.77 | 0.39–1.55 | 0.47 |
| CKD cause (diabetic nephropathy)† | 2.09 | 1.05–4.14 | 0.035 |
| Urinary protein (g/g Cr) | 0.94 | 0.79–1.12 | 0.46 |
| CKD stage 4‡ | 2.11 | 0.83–5.32 | 0.12 |
| AST-120§ | 0.51 | 0.26–0.99 | 0.046 |
References
1. Lee SW, Kim YC, Oh SW, Koo HS, Na KY, Chae DW, Kim S, Chin HJ. Trends in the prevalence of chronic kidney disease, other chronic diseases and health-related behaviors in an adult Korean population: data from the Korean National Health and Nutrition Examination Survey (KNHANES). Nephrol Dial Transplant 26:3975–3980. 2011;


2. ESRD Registry Committee, Korean Society of Nephrology. Current renal replacement therapy in Korea - Insan Memorial Dialysis Registry, 2015. In: The 36th Annual Meeting of the Korean Society of Nephrology; 2016; Seoul. Seoul: Korean Society of Nephrology; 2016.
3. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345:851–860. 2001;


4. Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snap-inn SM, Toto R. RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 63:1499–1507. 2003;


5. Hou FF, Xie D, Zhang X, Chen PY, Zhang WR, Liang M, Guo ZJ, Jiang JP. Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol 18:1889–1898. 2007;


6. Niwa T, Yazawa T, Maeda K, Ise M, Sugano M, Kodama T, Uehara Y. Effect of oral sorbent, AST-120, on serum concentration of indoxyl sulfate in uremic rats. Nihon Jinzo Gakkai Shi 32:695–701. 1990;

7. Lin CJ, Chen HH, Pan CF, Chuang CK, Wang TJ, Sun FJ, Wu CJ. p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J Clin Lab Anal 25:191–197. 2011;



8. Atoh K, Itoh H, Haneda M. Serum indoxyl sulfate levels in patients with diabetic nephropathy: relation to renal function. Diabetes Res Clin Pract 83:220–226. 2009;


9. Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S. The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int Suppl 62:S23–S28. 1997;

10. Niwa T. The protein metabolite theory as a mechanism for the progression of renal failure. J Ren Nutr 11:181–182. 2001;


11. Okada K, Takahashi S, Nagura Y, Hatano M, Shimamura T. Early morphological changes of tubules in rats with chronic renal failure. Nihon Jinzo Gakkai Shi 34:65–70. 1992;

12. Miyazaki T, Aoyama I, Ise M, Seo H, Niwa T. An oral sorbent reduces overload of indoxyl sulphate and gene expression of TGF-beta1 in uraemic rat kidneys. Nephrol Dial Transplant 15:1773–1781. 2000;


13. Horike K, Usami T, Kamiya Y, Kamiya T, Yoshida A, Itoh S, Yamato H, Ise M, Kimura G. Oral carbonaceous absorbent modifies renal function of renal ablation model without affecting plasma renin-angiotensin system or protein intake. Clin Exp Nephrol 7:120–124. 2003;


14. Takahashi N, Kawaguchi T, Suzuki T. Therapeutic effects of long-term administration of an oral adsorbent in patients with chronic renal failure: two-year study. Int J Urol 12:7–11. 2005;


15. Sanaka T, Akizawa T, Koide K, Koshikawa S. Protective effect of an oral adsorbent on renal function in chronic renal failure: determinants of its efficacy in diabetic nephropa-thy. Ther Apher Dial 8:232–240. 2004;


16. Shoji T, Wada A, Inoue K, Hayashi D, Tomida K, Furumatsu Y, Kaneko T, Okada N, Fukuhara Y, Imai E, Tsubakihara Y. Prospective randomized study evaluating the efficacy of the spherical adsorptive carbon AST-120 in chronic kidney disease patients with moderate decrease in renal function. Nephron Clin Pract 105:c99–c107. 2007;


17. Akizawa T, Asano Y, Morita S, Wakita T, Onishi Y, Fukuhara S, Gejyo F, Matsuo S, Yorioka N, Kurokawa K. CAP-KD Study Group. Effect of a carbonaceous oral adsorbent on the progression of CKD: a multicenter, randomized, controlled trial. Am J Kidney Dis 54:459–467. 2009;


18. Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Arita K, Kato A, Shimizu M. Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol 26:1732–1746. 2015;


19. Cha RH, Kang SW, Park CW, Cha DR, Na KY, Kim SG, Yoon SA, Han SY, Chang JH, Park SK, Lim CS, Kim YS. A randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Clin J Am Soc Nephrol 11:559–567. 2016;



20. Owada S, Goto S, Bannai K, Hayashi H, Nishijima F, Niwa T. Indoxyl sulfate reduces superoxide scavenging activity in the kidneys of normal and uremic rats. Am J Nephrol 28:446–454. 2008;


21. Gelasco AK, Raymond JR. Indoxyl sulfate induces complex redox alterations in mesangial cells. Am J Physiol Renal Physiol 290:F1551–F1558. 2006;


22. Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney Int 63:1671–1680. 2003;


23. Shimizu H, Bolati D, Adijiang A, Adelibieke Y, Muteliefu G, Enomoto A, Higashiyama Y, Higuchi Y, Nishijima F, Niwa T. Indoxyl sulfate downregulates renal expression of Klotho through production of ROS and activation of nuclear factor-κB. Am J Nephrol 33:319–324. 2011;


24. Owada A, Nakao M, Koike J, Ujiie K, Tomita K, Shiigai T. Effects of oral adsorbent AST-120 on the progression of chronic renal failure: a randomized controlled study. Kidney Int Suppl 63:S188–S190. 1997.

25. Ueda S, Yamagishi S, Takeuchi M, Kohno K, Shibata R, Mat-sumoto Y, Kaneyuki U, Fujimura T, Hayashida A, Okuda S. Oral adsorbent AST-120 decreases serum levels of AGEs in patients with chronic renal failure. Mol Med 12:180–184. 2006;



26. Adijiang A, Niwa T. An oral sorbent, AST-120, increases Klotho expression and inhibits cell senescence in the kidney of uremic rats. Am J Nephrol 31:160–164. 2010;


27. Maeda K, Hamada C, Hayashi T, Shou I, Wakabayashi M, Fukui M, Horikoshi S, Tomino Y. Long-term effects of the oral adsorbent, AST-120, in patients with chronic renal failure. J Int Med Res 37:205–213. 2009;


28. Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol 289:F645–F659. 2005;


29. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 65:2309–2320. 2004;


30. Goto S, Kitamura K, Kono K, Nakai K, Fujii H, Nishi S. Association between AST-120 and abdominal aortic calcification in predialysis patients with chronic kidney disease. Clin Exp Nephrol 17:365–371. 2013;





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print
















