| Kidney Res Clin Pract > Volume 36(1); 2017 > Article |
|
Abstract
Background
Previous studies have shown that a higher resistive index (RI) on renal duplex ultrasonography was related with renal progression and acute kidney injury, especially in patients with chronic kidney disease (CKD) using an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor antagonist (ARB). We evaluated whether a RI value is a predictive factor for renal progression regardless of ACEI or ARB medication in patients with moderate renal dysfunction.
Methods
We retrospectively analyzed 119 patients with moderate renal dysfunction that had been evaluated with renal duplex ultrasonography from February 2011 to April 2015. Moderate renal dysfunction was defined as a stage 3 to 4 CKD. Renal progression was defined as a doubling of the baseline serum creatinine (sCr), a decrease of baseline glomerular filtration rate by > 50%, or initiation of renal replacement therapy.
Results
The mean age was 64.7 ┬▒ 11.0 years and sCr level was 2.1 ┬▒ 1.2 mg/dL. The RI Ōēź 0.79 group showed a higher incidence of renal progression (P = 0.004, log-rank test) compared with the RI < 0.79 group, irrespective of ACEI or ARB usage. In the Cox proportional hazard model, RI Ōēź 0.79 was an independent prognostic factor after adjusting for age, sex, diabetes mellitus, sCr, proteinuria, and use of ACEI or ARB (hazard ratio, 4.88; 95% confidence interval, 1.06ŌĆō22.53; P = 0.043).
Chronic kidney disease (CKD) is a major public health problem, and the incidence and prevalence of CKD have been increasing annually. Patients with CKD are at a high risk of not only renal progression but also cardiovascular disease (CVD) [1,2]. Several biomarkers, including serum creatinine (sCr), proteinuria, and neutrophil gelatinase-associated lipocalin are commonly used as predictors of renal prognosis in patients with CKD [3]. However, it is still difficult to predict renal outcome precisely because multiple factors, such as control of hypertension and renal ischemia due to renal atherosclerosis, affect renal progression.
It is well known that use of an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor antagonist (ARB) can retard renal progression [4]. These medications have been commonly used in clinical practice not only to control hypertension but also to reduce protein-uria. On the contrary, it is of note that these strategies can increase the risk of acute kidney injury (AKI) or hyperkalemia [5,6]. AKI is a well-known important risk factor of renal progression in patients with CKD [7]. Therefore, patients who have not taken ACEI or ARB due to AKI are at risk of renal progression. There is no study evaluating factors for renal progression among CKD patients who cannot take ACEI or ARB.
The renal resistive index (RI) is a measure of renal arterial resistances to blood flow detected by kidney doppler ultrasonography. RI appears to be a good indicator of renal vascular resistance [8,9]. RI was also well-correlated with renal arteriolosclerosis [10]. A few previous studies have reported that higher RI values are associated with renal progression [11ŌĆō13]. However, studies regarding the relationship between RI values and renal prognosis in patients with moderate renal dysfunction are limited.
The objective of this study was to assess the role of the RI measured with the initial sCr level on predicting renal progression or initiation of renal replacement therapy (RRT) in patients with moderate renal dysfunction. In addition, we investigated the relationship between ACEI or ARB usage and renal progression with respect to RI values.
This single center study was conducted from January 2011 to April 2015. We screened 146 patients who had been diagnosed with stage 3 or 4 CKD, were simultaneously evaluated with renal duplex ultrasonography, and were over 18 years of age. The criteria for exclusion were the following: patients with a single kidney, patients undergoing RRT, patients with renal artery stenosis, or patients with clinical evidence of renovascular stenosis. Patients who were followed up for at least 24 months were enrolled. Ultimately, 119 patients were included in this study.
We retrospectively analyzed the patientsŌĆÖ medical records, age, sex, blood pressure, and other comorbidities such as diabetes mellitus (DM), hypertension, ischemic heart disease (IHD), and cerebrovascular accidents (CVA). Renal function was evaluated using sCr, estimated glomerular filtration rate (eGFR), cystatin-c, and cystatin-c-based GFR. Estimated GFR was calculated using the Modified Diet in Renal Disease equation (MDRD) and CKD Epidemiology Collaboration (CKD-EPI) equation [14,15]. CKD was defined according to National Kidney FoundationŌĆÖs Kidney Disease Outcomes Quality Initiative (NKF-K/DOQI) guideline in 2002 and moderate renal dysfunction was defined as a stage 3 to 4 CKD [16]. Renal progression was defined as a doubling of baseline sCr, a decrease of baseline GFR by > 50%, or initiation of RRT [17ŌĆō19]. AKI caused by ACEI or ARB treatment was defined as showing any one criterion of two diagnosis criteria in previous study [13]. First, sCr was elevated more than 30% of baseline sCr within 1 month of taking ACEI or ARB. Second, sCr level was slowly elevated more than 30% of baseline sCr within 3 months without other cause or sCr was rapidly recovered more than 30% after stopping ACEI or ARB and persistently maintained sCr for > 3 months. Cardiovascular events were defined as the occurrence of IHD, CVA, or peripheral artery disease during the follow-up period.
This study was approved by the Dong-A University Hospital Institutional Review Board (DAUHIRB-16-002). Informed consent was waived because the study was of a retrospective design and the data were analyzed anonymously. The study was conducted in accordance with the Declaration of Helsinki.
Renal duplex ultrasonography was performed by one radiologist using a Siemens Sequoia 512 (Siemens Medical Solutions USA, Issaquah, WA, USA). The renal RI was calculated as [1 ŌĆō (end diastolic frequency shift/peak systolic frequency shift)] using doppler samples from the segmental and interlobar arteries of the kidney with the higher main renal artery peak systolic velocity. Using a 3-mm Doppler sample, the parenchymal peak systolic and end diastolic frequency shifts (kHz) of the segmental and interlobar arteries were obtained.
The data are presented as the mean ┬▒ standard deviation or frequency. The subjectsŌĆÖ characteristics were analyzed by using StudentŌĆÖs t-test for continuous variables, such as age, duration for follow up, baseline renal function, and systemic factors. The chi-squared test for categorical variables, such as medical history, medication, and clinical outcomes were performed between two groups. The area under the receiver operating characteristic (ROC) curve was used to determine cut-off value of RI for the prediction of renal progression. Kaplan-Meier analysis and log-rank tests were performed to demonstrate the cumulative risk of renal progression. Furthermore, a multivariate Cox proportional hazards regression analysis was performed to clarify the factors associated with renal progression. The P-value less than 0.05 was considered to be statistically significant. All statistical calculations were performed with PASW Statistics software (version 18.0; IBM Co., Armonk, NY, USA).
The patient characteristics are summarized in Table 1. The mean patient age was 64.7 ┬▒ 11.0 years, and 60.5% were male. Of the enrolled patients, 84.9% had hypertension, 42.9% had DM, 8.4% had IHD, and 14.3% had CVA. The mean sCr level was 2.1 ┬▒ 1.2 mg/dL, and the mean RI was 0.79 ┬▒ 0.08. Sixty patients (50.4%) were treated with an ACEI or ARB and their mean RI was 0.75 ┬▒ 0.09.
We stratified the patients into two groups depending on whether they received ACEIs or ARBs, or not (Table 1). There were no significant differences in baseline renal function between both groups. Most patients treated without an ACEI or ARB had a previous history of AKI caused by ACEI or ARB treatment. The RI value was significantly higher in patients treated without ACEI or ARB than in those treated with ACEI or ARB (P = 0.002). However, there was no difference in the size of the kidneys. The proportion of patients with renal progression in the group treated with ACEIs or ARBs was lower than that of those treated without ACEIs or ARBs (18.3% vs. 40.7%, P = 0.007).
We analyzed the diagnostic performance of the RI value for the prediction of renal progression (Fig. 1). The area under the ROC curve was 0.705 (95% confidence interval [CI], 0.609ŌĆō0.801; P < 0.001), and a renal RI Ōēź 0.79 predicted renal progression with 82.9% sensitivity and 51.2% specificity.
Patients with RI Ōēź 0.79 were older and commonly had a history of DM and IHD (Table 2). The sCr levels were higher in patients with RI Ōēź 0.79 than in patients with RI < 0.79 (P = 0.001). The eGFR, hemoglobin, and albumin levels were significantly lower in patients with RI Ōēź 0.79 than they were in those with RI < 0.79 (P < 0.001, P = 0.001, and P = 0.002, respectively). The proportion of patients with renal progression in the group with RI Ōēź 0.79 was higher than that of those with RI < 0.79 (41.9% vs. 8.9%, P < 0.001). In addition, the patients with RI Ōēź 0.79 had a tendency for increased number of cardiovascular events compared with those with RI < 0.79 (P = 0.051, log-rank test).
Of all patients treated with ACEIs or ARBs, 27 patients (45.0%) had RI Ōēź 0.79 on renal duplex ultrasonography (Table 3). Among them, patients with RI Ōēź 0.79 had a greater incidence of DM or IHD than those with RI < 0.79. The eGFR, hemoglobin, and albumin levels were significantly lower in patients with RI Ōēź 0.79 than in those with an RI < 0.79. Among the patients treated without ACEIs or ARBs, 47 patients (79.7%) had RI Ōēź 0.79 on renal duplex ultrasonography. Higher renal progression was found in the patients with RI Ōēź 0.79 regardless of ACEI or ARB usage.
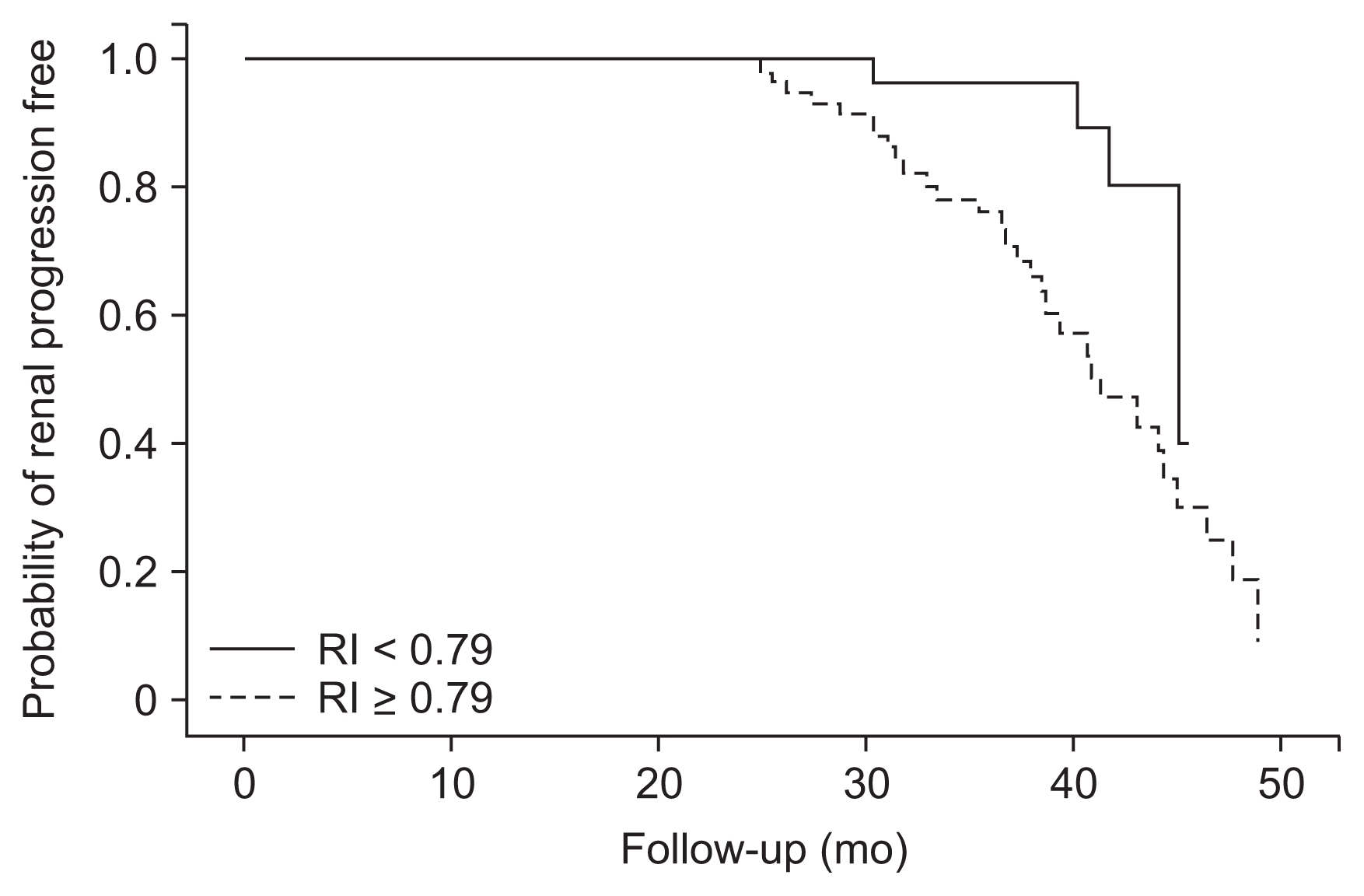
The patients with RI Ōēź 0.79 had significantly higher incidence of renal progression than those with RI < 0.79 (P = 0.004, log-rank test; Fig. 2). In Cox proportional hazards regression analysis, RI Ōēź 0.79 was an independent prognostic factor after adjustment for age, sex, DM, sCr, proteinuria, and use of ACEIs or ARBs (hazard ratio, 4.88; 95% CI, 1.06ŌĆō22.53; P = 0.043; Table 4).
One previous study showed that RI > 0.8 on renal duplex ultrasonography was a predictor of worsened renal function and progression to renal replacement in patients newly diagnosed with CKD [11]. Another observational study also demonstrated that RI > 0.7 was an independent risk factor for the progression of CKD [12]. Present study also showed that RI Ōēź 0.79 can predict renal progression in patients with moderate renal dysfunction. These studies support that higher RI > 0.7 may be related with renal progression. Similar to the present study, patients with a higher RI had a higher rate of DM (e.g., 40% vs. 8%), lower renal function (e.g., creatinine clearance, 24 ┬▒ 16 mL/min/1.73m2 vs. 91 ┬▒ 31 mL/min/1.73 m2), and were older (e.g., 66 ┬▒ 10 years vs. 47 ┬▒ 16 years) compared with those with a lower RI in the previous two studies [11,12]. However, the enrolled patients had a mean age > 60 years, mean RI values > 7.0, GFR < 60 mL/min/1.73 m2, and a prevalence of diabetes > 40% in this study. Therefore, the cut off value of RI for predicting rapid renal progression may be 0.79 in elderly patients (> 60 years) with moderate renal dysfunction. The cut off value of RI for predicting rapid renal progression may be 0.7 in younger patients (Ōēż 60 years) with renal dysfunction. It is not conclusive which value of RI is the more important cutoff point because of a lack of studies. Further prospective studies are necessary to demonstrate a clear cutoff value for RI in CKD patients.
Kidney ultrasonography should be performed to estimate kidney size, structural abnormalities, and cortical echogenicity of the kidneys in nearly all cases of suspected CKD. Measuring the RI gives additional information outside of kidney size and echogenicity when kidney ultrasonography is performed. Although renal pathology was not included in this study, the RI is significantly higher in nephropathies with tubulointerstitial and/or vascular injury, although the mechanism is not clear [20,21]. Alterations in the postglomerular vessels due to interstitial fibrosis can increase resistance to renal cortical blood flow, subsequently reducing glomerular perfusion [22]. A higher RI also implies target organ damage in essential hypertension [9]. Atherosclerosis in diabetic patients can be assumed by RI elevation on renal duplex ultrasonography [23]. These results suggest that a higher RI indirectly reflects atherosclerosis, target organ damage, tubulointerstitial injury or fibrosis, and vascular injury without pathologic evaluation. Based on our results, a higher RI is related not only with renal progression but also cardiovascular events and prevalence of IHD. Kawai et al [24] also showed that the RI may be a useful marker to detect and evaluate atherosclerotic diseases. Therefore, we recommend that routine checking of the RI using renal duplex ultrasonography may provide additional information in predicting renal progression in patients with CKD. Further studies are necessary to evaluate the role of the initial RI value and serial change in RI while checking for sCr increase.
The prevalence of diabetes and number of elderly patients are sharply increasing [25]. These phenomena result in increasing atherosclerosis-related disease. Atherosclerotic renovascular disease is also common [26]. Recent clinical trials in patients with atherosclerotic renovascular disease found that renal revascularization was not superior to medical therapy using ACEIs or ARBs [27ŌĆō29]. There is no significant decrease in renal blood flow in atherosclerotic renovascular disease if vascular occlusions are less than 50ŌĆō60% [30]. However, there are no definite markers of or non-invasive studies reflecting the amount of arterial occlusion without angiography. In the future, the RI value on renal duplex ultrasonography associated with atherosclerosis and renal artery resistance may reflect severity of arterial occlusion in patients with CKD. Further studies are necessary to demonstrate this role of the RI value.
Treatment with ACEIs or ARBs is known to reduce the progression of CKD [4]. In this study, patients treated with ACEIs or ARBs seemed to have a better renal prognosis. Most patients treated without ACEIs or ARBs were initially treated with them. However, the medication was stopped because of the occurrence of AKI. These patients had higher mean RI values and a higher percentage of diuretic use compared with patients continuously treated with ACEIs or ARBs. RI > 0.8 can predict AKI risk after the use of ACEI or ARB medication based on our previous study [13]. The benefit of ACEIs or ARBs in patients with CKD without use of them was not apparent when considering renal progression. In this study, we demonstrated that a higher RI value was a significant risk factor for renal progression. Especially, the risk of renal progression was not high in CKD patients with a low RI value, regardless of their ACEI or ARB usage.
This study has several limitations. First, the sample size is small and the study was performed retrospectively. Second, most enrolled patients who were not being treated with ACEIs or ARBs had a history of AKI due to their use. This bias of enrolled patients may have had an effect on the results. Despite these limitations, we observed that RI Ōēź 0.79 predicted higher incidence of renal progression in patients with CKD with moderate renal dysfunction, regardless of their current ACEI or ARB usage.
In conclusion, RI Ōēź 0.79 on the renal duplex ultrasonography can be a helpful predictor for renal progression in patients with moderate renal dysfunction, regardless of their ACEI or ARB usage. Therefore, checking the RI value is helpful when we evaluate kidney ultrasonography in patients with moderate renal dysfunction.
Figure┬Ā1
The receiver-operating characteristics (ROC) curve for the prediction of renal progression
The area under the ROC curve was 0.705 (95% confidence interval, 0.609ŌĆō0.801, P < 0.001), and a renal resistive index Ōēź 0.79 predicted renal progression with 82.9% sensitivity and 51.2% specificity.

Figure┬Ā2
Kaplan-Meier survival curves of renal progression
The patients with resistive index (RI) value Ōēź 0.79 had a significantly higher incidence of renal progression compared with those with RI value < 0.79 (P = 0.005, log-rank test).
Table┬Ā1
Clinical characteristics of patients with and without ACEI or ARB
Table┬Ā2
Comparison of clinical characteristics in accordance with resistive index (RI) value
Table┬Ā3
Clinical characteristics of patients with and without ACEI or ARB
| Characteristic | Without ACEI or ARB | With ACEI or ARB | ||
|---|---|---|---|---|
|
|
|
|||
| RI < 0.79 (n = 12) | RI Ōēź 0.79 (n = 47) | RI < 0.79 (n = 33) | RI Ōēź 0.79 (n = 27) | |
| Age (yr) | 64.1 ┬▒ 13.0 | 66.3 ┬▒ 10.2 | 60.2 ┬▒ 11.7 | 67.6 ┬▒ 9.4* |
| Male | 6 (50.0) | 30 (63.8) | 18 (54.5) | 18 (66.7) |
| Duration for follow up (mo) | 32.6 ┬▒ 6.1 | 35.3 ┬▒ 7.0 | 35.2 ┬▒ 6.8 | 33.6 ┬▒ 6.6 |
| Medical history | ||||
| ŌĆāDiabetes mellitus | 3 (25.0) | 23 (48.9) | 6 (18.2) | 19 (70.4)* |
| ŌĆāHypertension | 7 (58.3) | 39 (83.0)* | 30 (90.9)ŌĆĀ | 25 (92.6) |
| ŌĆāIschemic heart disease | 0 | 5 (10.6) | 0 | 5 (18.5)* |
| ŌĆāCerebrovascular accidents | 1 (8.3) | 5 (10.6) | 4 (12.1) | 7 (25.9) |
| ŌĆāChronic kidney disease | ||||
| ŌĆāŌĆāStage 3 | 10 (83.3) | 23 (48.9) | 24 (72.7) | 15 (55.6) |
| ŌĆāŌĆāStage 4 | 2 (16.7) | 24 (51.1)* | 9 (27.3) | 12 (44.4) |
| Baseline renal function | ||||
| ŌĆāCreatinine (mg/dL) | 1.7 ┬▒ 0.4 | 2.3 ┬▒ 0.8* | 1.8 ┬▒ 0.5 | 2.5 ┬▒ 2.1 |
| ŌĆāRUP/Cr (g/g) | 1.2 ┬▒ 2.6 | 3.1 ┬▒ 4.2 | 1.8 ┬▒ 2.9 | 1.8 ┬▒ 2.7 |
| ŌĆāGFR (mL/min/1.73 m2) | 41.5 ┬▒ 10.0 | 31.8 ┬▒ 11.7* | 40.9 ┬▒ 12.6 | 31.1 ┬▒ 10.1* |
| ŌĆāCKD-EPI GFR | 34.0 ┬▒ 9.3 | 24.9 ┬▒ 9.4* | 33.7 ┬▒ 9.8 | 24.7 ┬▒ 8.5* |
| ŌĆāCystatin C (mg/dL) | 1.7 ┬▒ 0.5 | 2.2 ┬▒ 0.6* | 1.9 ┬▒ 0.6 | 2.1 ┬▒ 0.5 |
| ŌĆāCystatin C based GFR (mL/min/1.73 m2) | 42.4 ┬▒ 15.2 | 31.8 ┬▒ 10.3 | 36.7 ┬▒ 12.2 | 32.5 ┬▒ 9.1 |
| Renal duplex ultrasonography | ||||
| ŌĆāRight kidney size (cm) | 10.0 ┬▒ 1.1 | 10.6 ┬▒ 1.3 | 10.4 ┬▒ 2.1 | 10.3 ┬▒ 2.0 |
| ŌĆāRI | 0.72 ┬▒ 0.05 | 0.85 ┬▒ 0.04* | 0.71 ┬▒ 0.06 | 0.85 ┬▒ 0.04* |
| Systemic factor | ||||
| ŌĆāHemoglobin (g/dL) | 11.3 ┬▒ 1.7 | 11.2 ┬▒ 2.2 | 13.1 ┬▒ 1.9ŌĆĀ | 11.5 ┬▒ 1.5* |
| ŌĆāAlbumin (g/dL) | 4.1 ┬▒ 0.4 | 3.9 ┬▒ 0.6 | 4.3 ┬▒ 0.4 | 4.1 ┬▒ 0.3* |
| ŌĆāSystolic BP (mmHg) | 118.8 ┬▒ 17.4 | 132.3 ┬▒ 17.5* | 129.6 ┬▒ 12.9ŌĆĀ | 136.9 ┬▒ 16.8 |
| ŌĆāDiastolic BP (mmHg) | 69.5 ┬▒ 16.8 | 70.7 ┬▒ 11.4 | 76.4 ┬▒ 13.3 | 71.6 ┬▒ 13.3 |
| ŌĆāPulse pressure (mmHg) | 49.3 ┬▒ 14.8 | 61.5 ┬▒ 19.1* | 53.1 ┬▒ 14.8 | 65.2 ┬▒ 12.6* |
| Medication | ||||
| ŌĆāBeta blocker | 1 (8.3) | 20 (42.6)* | 7 (21.2) | 7 (25.9) |
| ŌĆāCalcium channel blocker | 5 (41.7) | 28 (59.6) | 18 (54.5) | 17 (63.0) |
| ŌĆāFurosemide | 2 (16.7) | 14 (29.8) | 2 (6.1) | 5 (18.5) |
| ŌĆāSpironolactone | 1 (8.3) | 5 (10.6) | 0 | 3 (11.1)* |
| ŌĆāDigoxin | 0 | 10 (21.3) | 1 (3.0) | 2 (7.4) |
| ŌĆāStatin | 5 (41.7) | 23 (48.9) | 19 (57.6) | 15 (55.6) |
| Renal progression | 2 (16.7) | 22 (46.8)* | 2 (6.1) | 9 (33.3)* |
| ŌĆāCreatinine doubling or GFR < 50% | 0 | 9 (19.1) | 1 (3.0) | 5 (18.5)* |
| ŌĆāEnd-stage renal disease | 2 (16.7) | 13 (27.7) | 1 (3.0) | 4 (14.8) |
| Cardiovascular event | 0 | 4 (8.5) | 0 | 2 (7.4) |
Table┬Ā4
Independent factors associated with renal progression
References
1. Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13:745ŌĆō753. 2002;


2. Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164:659ŌĆō663. 2004;


3. Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4:337ŌĆō344. 2009;



4. Ripley E. Complementary effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in slowing the progression of chronic kidney disease. Am Heart J 157(6 Suppl):S7ŌĆōS16. 2009;


5. Raebel MA. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther 30:e156ŌĆōe166. 2012;


6. Wang AY, Bellomo R, Ninomiya T, Lo S, Cass A, Jardine M, Gallagher M. Angiotensin-converting enzyme inhibitor usage and acute kidney injury: a secondary analysis of RENAL study outcomes. Nephrology (Carlton) 19:617ŌĆō622. 2014;


7. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20:223ŌĆō228. 2009;



8. Raff U, Schmidt BM, Schwab J, Schwarz TK, Achenbach S, B├żr I, Schmieder RE. Renal resistive index in addition to low-grade albuminuria complements screening for target organ damage in therapy-resistant hypertension. J Hypertens 28:608ŌĆō614. 2010;


9. Florczak E, Januszewicz M, Januszewicz A, Prejbisz A, Kaczmarska M, Micha┼éowska I, Kabat M, Rywik T, Rynkun D, Zieli┼äski T, Ku┼ømierczyk-Droszcz B, Pregowska-Chwa┼éa B, Kowalewski G, Hoffman P. Relationship between renal resistive index and early target organ damage in patients with never-treated essential hypertension. Blood Press 18:55ŌĆō61. 2009;


10. Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, Moriya H, Suzuki S, Miura S. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis 46:603ŌĆō609. 2005;


11. Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension 39:699ŌĆō703. 2002;


12. Sugiura T, Wada A. Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transplant 24:2780ŌĆō2785. 2009;


13. Kim ES, Kim HJ, Kim YJ, Lee SM, Lee HJ, Cho DS, Son YK, Kim SE, Kim KH, An WS. Resistive index as a predictor of acute kidney injury caused by an angiotensin converting enzyme inhibitor or angiotensin II receptor blocker in chronic kidney disease patients. Kidney Res Clin Pract 32:158ŌĆō163. 2013;



14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604ŌĆō612. 2009;



15. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247ŌĆō254. 2006;


16. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39(2 Suppl 1):S1ŌĆōS266. 2002;

17. Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305:1553ŌĆō1559. 2011;


18. Lambers Heerspink HJ, Perkovic V, de Zeeuw D. Is doubling of serum creatinine a valid clinical ŌĆśhardŌĆÖ endpoint in clinical nephrology trials? Nephron Clin Pract 119:c195ŌĆōc199. discussion c199. 2011


19. Inaguma D, Imai E, Takeuchi A, Ohashi Y, Watanabe T, Nitta K, Akizawa T, Matsuo S, Makino H, Hishida A. Risk factors for CKD progression in Japanese patients: findings from the Chronic Kidney Disease Japan Cohort (CKD-JAC) study. Clin Exp Nephrol Epub 2016 Jul 13.

20. Big├® N, L├®vy PP, Callard P, Faintuch JM, Chigot V, Jousselin V, Ronco P, Boffa JJ. Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic kidney disease. BMC Nephrol 13:1392012;



21. Parolini C, Noce A, Staffolani E, Giarrizzo GF, Costanzi S, Splendiani G. Renal resistive index and long-term outcome in chronic nephropathies. Radiology 252:888ŌĆō896. 2009;


22. Bader R, Bader H, Grund KE, Mackensen-Haen S, Christ H, Bohle A. Structure and function of the kidney in diabetic glomerulosclerosis. Correlations between morphological and functional parameters. Pathol Res Pract 167:204ŌĆō216. 1980;

23. Milovanceva-Popovska M, Dzikova S. Progression of diabetic nephropathy: value of intrarenal resistive index (RI). Prilozi 28:69ŌĆō79. 2007;

24. Kawai T, Kamide K, Onishi M, Yamamoto-Hanasaki H, Baba Y, Hongyo K, Shimaoka I, Tatara Y, Takeya Y, Ohishi M, Rakugi H. Usefulness of the resistive index in renal Doppler ultrasonography as an indicator of vascular damage in patients with risks of atherosclerosis. Nephrol Dial Transplant 26:3256ŌĆō3262. 2011;


25. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301:2129ŌĆō2140. 2009;


26. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405ŌĆō412. 2000;



27. Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, DŌĆÖAgostino RB Sr, Dworkin LD. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370:13ŌĆō22. 2014;


28. ASTRAL Investigators. Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361:1953ŌĆō1962. 2009;


29. Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 150:840ŌĆō848. W150ŌĆōW151. 2009;





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















